The Golgi apparatus stands as one of the most fascinating organelles in eukaryotic cells, acting like a bustling factory that processes, sorts, and ships essential molecules. Discovered over a century ago, this structure plays a pivotal role in maintaining cellular health and function. From modifying proteins to synthesizing complex carbohydrates, its contributions are vital for everything from hormone production to cell wall formation in plants.
In this in-depth article, we’ll explore its discovery, intricate structure, diverse functions, and variations across organisms, drawing on established scientific insights to provide a thorough understanding. Whether you’re a student, researcher, or simply curious about cell biology, this guide breaks down the complexities in straightforward terms, with examples, tables, and detailed explanations to illustrate its significance.
Table of Contents
Discovery and History of the Golgi Apparatus
The story of the Golgi apparatus begins in the late 19th century with Italian physician and biologist Camillo Golgi. In 1898, while studying the nervous system using a silver staining technique he developed, Golgi observed a thread-like network inside cells that he called the “internal reticular apparatus.”
At first, many scientists dismissed it as an artifact or optical illusion created by the staining method, sparking debates that lasted for decades. It wasn’t until the advent of electron microscopy in the mid-20th century that the organelle’s existence was definitively confirmed. By the 1950s, researchers like George Palade used these advanced tools to visualize its stacked membrane structure, solidifying its place in cell biology.
The name “Golgi apparatus” was coined in 1910, honoring its discoverer, and it appeared in scientific literature by 1913. Over time, terms like “Golgi complex” emerged in 1956 to describe its multifaceted nature. This historical journey highlights how technological advancements, from light microscopy to electron and fluorescence imaging, have deepened our appreciation of cellular components. Today, live-cell imaging techniques allow us to observe the Golgi in action, revealing its dynamic behavior in real time. This evolution in understanding underscores the organelle’s central role in eukaryotic life, bridging early observations with modern molecular biology.
Interestingly, the initial skepticism around Golgi’s discovery mirrors broader challenges in early cytology, where distinguishing real structures from preparation artifacts was common. As research progressed, the Golgi’s functions became clearer, linking it to secretory pathways essential for multicellular organisms. For instance, in nerve cells the organelle helps package neurotransmitters, directly tying back to Golgi’s original focus on the nervous system.
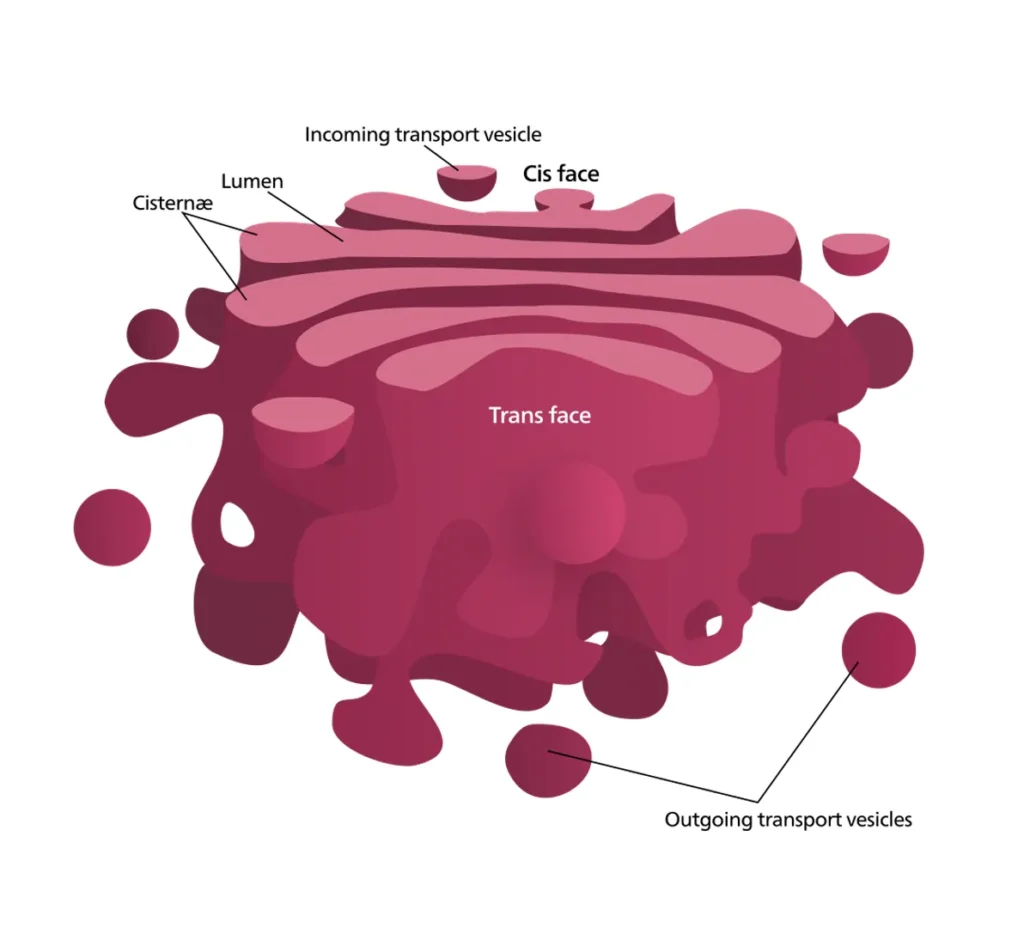
What is the Golgi Apparatus?
At its core, the Golgi apparatus is a key organelle found in most eukaryotic cells, from animals and plants to fungi and protists. It consists of a series of flattened, membrane-bound sacs known as cisternae, stacked together like pancakes to form what is often called the Golgi stack or Golgi complex. Typically located near the nucleus and the endoplasmic reticulum (ER), it serves as a processing and distribution center for proteins and lipids synthesized in the cell.
In animal cells, you might find 10 to 20 Golgi stacks per cell, interconnected by tubular structures, while plant cells feature numerous smaller units called dictyosomes scattered throughout the cytoplasm. The organelle is absent in certain cells, such as mature red blood cells in mammals, sperm cells, bacteria, and blue-green algae, where secretory needs are minimal or handled differently. Its size and shape can vary based on the cell’s activity level; for example, in highly secretory cells like those in the pancreas, the Golgi expands to handle increased protein packaging.
The Golgi has distinct polarity, with a cis face (entry side) facing the ER to receive incoming materials and a trans face (exit side) where processed molecules are packaged into vesicles for delivery. This directional flow ensures efficient processing, much like an assembly line. In single-celled organisms like some protists, the Golgi can have up to 60 cisternae, adapting to their unique metabolic demands. Overall, the Golgi’s presence is a hallmark of eukaryotic complexity, enabling sophisticated cellular communication and maintenance.
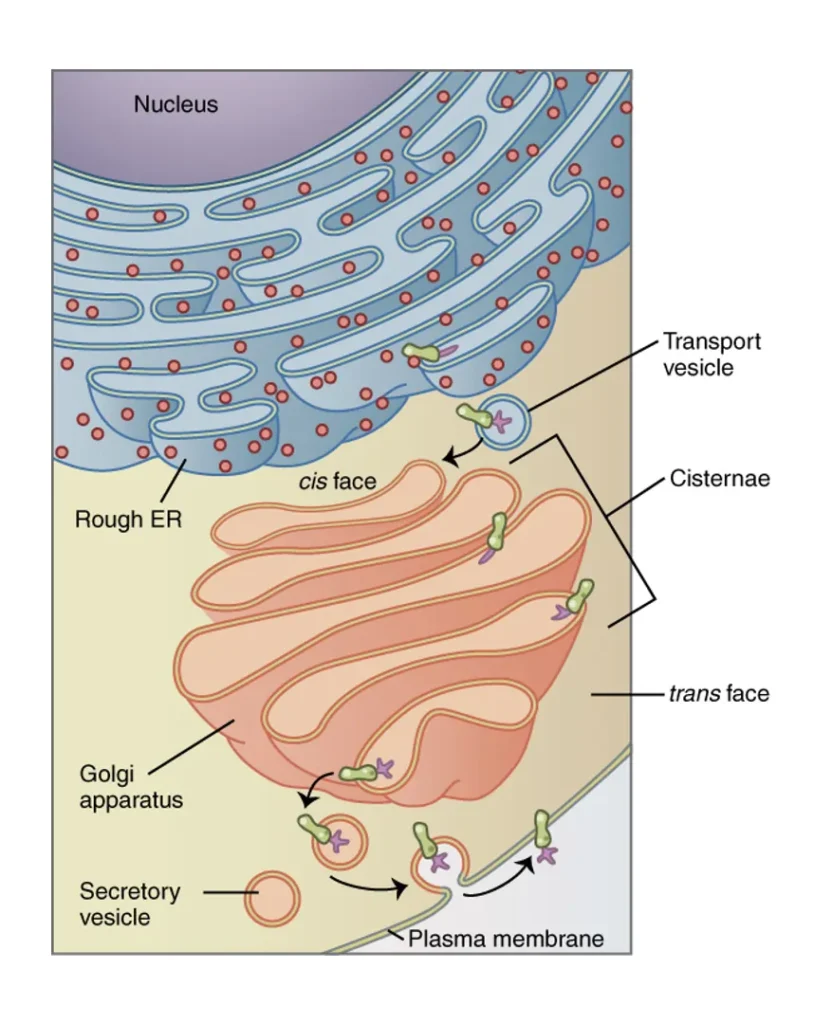
Structure of the Golgi Apparatus
The Golgi apparatus boasts a highly organized structure tailored for its processing roles. Primarily composed of cisternae – flat, disc-shaped sacs measuring about 0.5 to 1.0 micrometers in diameter – these are stacked parallel to one another, usually in groups of four to eight in most cells. The stacks are held together by a protein matrix and interconnected tubules, creating a compact yet flexible unit.
Surrounding the cisternae are smaller components like tubules, which branch out to connect different parts, vesicles that bud off for transport, and vacuoles that store processed materials. The entire structure is polarized: the convex cis face (also called the forming face) is closest to the ER, receiving transport vesicles, while the concave trans face (maturing face) faces away, releasing secretory vesicles. Between these are medial cisternae, where much of the modification occurs.
This architecture allows for sequential processing as molecules move from cis to trans. In mammals, microtubules anchor the Golgi near the centrosome, aiding stability, whereas in plants, actin filaments enable mobility. The trans Golgi network (TGN) acts as a sorting hub at the exit, sometimes functioning as an early endosome in plants and yeast. Variations exist; for example, some yeast species lack stacked cisternae entirely, opting for scattered compartments.
To visualize this better, consider the following table outlining the main structural components:
| Component | Description | Role in Structure |
|---|---|---|
| Cisternae | Flattened, membrane-bound sacs stacked in parallel | Form the core of the Golgi stack; site for enzymatic modifications |
| Tubules | Branching extensions connecting cisternae | Facilitate material transfer between stacks and maintain connectivity |
| Vesicles | Small, spherical membrane bubbles | Bud off from edges for transporting proteins and lipids to and from the Golgi |
| Vacuoles | Larger storage compartments | Hold processed macromolecules before release or further use |
| Cis Face | Convex entry side oriented toward the ER | Receives incoming vesicles from the ER for initial processing |
| Trans Face | Concave exit side | Releases modified vesicles to destinations like lysosomes or the plasma membrane |
| Trans Golgi Network (TGN) | Mesh-like extension at the trans end | Sorts and packages molecules into specific vesicles for targeted delivery |
This table highlights how each part contributes to the organelle’s efficiency. The structure’s adaptability is evident in how it responds to cellular stress, sometimes fragmenting or enlarging based on needs.
Diagram of the Golgi Apparatus
Imagine a detailed diagram of the Golgi apparatus: at the center, a stack of five to eight curved, flattened cisternae resembling a pile of slightly bowed plates. The cis face is depicted on the left, convex and facing a cluster of small vesicles arriving from the rough ER, shown as dotted arrows indicating flow. Enzymes within the cisternae are illustrated as tiny symbols, with progressive changes in color representing modifications like glycosylation.
Moving rightward, the medial cisternae show intermediate processing, while the trans face on the right is concave, with larger vesicles budding off toward the plasma membrane, lysosomes, or other organelles. Tubules connect adjacent stacks, and the TGN appears as a network of tubules and vesicles at the trans end. Labels point out key parts, and a magnified inset details a cisterna’s membrane with embedded proteins. This visual representation emphasizes the directional traffic, underscoring the Golgi’s role as a cellular highway.
Functions of the Golgi Apparatus
The Golgi apparatus performs a multitude of functions critical to cellular operations, acting as the cell’s post office and modification center. One primary role is protein modification, where it adds carbohydrates (glycosylation), phosphates, or sulfates to proteins arriving from the ER. For example, in the formation of glycoproteins, sugars like mannose are trimmed and others like sialic acid added, ensuring proper folding and function.
It also handles lipid processing, synthesizing molecules like sphingomyelin and glycolipids, which are essential for cell membrane integrity and signaling. In endocrine cells, the Golgi packages hormones into vesicles for release, such as insulin in pancreatic beta cells. Additionally, it contributes to lysosome formation by directing enzymes to these digestive organelles, aiding in waste breakdown.
Here are some key functions in bullet points for clarity:
- Glycosylation of Proteins: Attaches sugar chains to proteins, creating glycoproteins vital for cell recognition, as seen in blood type determinants on red blood cells.
- Lipid Synthesis and Modification: Produces glycolipids for membrane asymmetry, with polar heads exposed on the cell surface for interactions.
- Sorting and Packaging: Directs molecules into vesicles for secretion, storage, or organelle delivery, like packaging digestive enzymes for lysosomes.
- Formation of Connective Tissue Matrix: In fibroblasts, it assembles proteoglycans for extracellular matrix support.
- Hormone Production: Modifies and packages hormones in glands, ensuring timely release in response to signals.
- Root Hair Formation: In plant root cells, it helps synthesize materials for hair extension, aiding nutrient absorption.
- Membrane Recycling: Transforms and recycles cellular membranes, maintaining lipid balance.
These functions illustrate the Golgi’s versatility. In chick embryos, for instance, it synthesizes retinal pigments, demonstrating its role in development.
Role in Protein and Lipid Processing
Diving deeper, the Golgi’s processing of proteins involves sequential enzymatic reactions across its compartments. N-linked glycosylation starts in the ER but is refined in the Golgi: mannose residues are removed in the cis Golgi, N-acetylglucosamine added in the medial Golgi, and galactose or sialic acid in the trans Golgi. O-linked glycosylation, attaching sugars to serine or threonine, occurs entirely here.
For lipids, ceramide from the ER is converted to sphingomyelin on the lumenal surface or glycolipids flipped across membranes. This processing ensures lipids end up in the correct membrane leaflet, with head groups outward for cell signaling. In lysosomal targeting, mannose-6-phosphate tags are added to enzymes, recognized by receptors in the TGN for vesicle sorting.
Examples abound: in immune cells, the Golgi modifies antibodies for effective pathogen binding. Disruptions can lead to improper protein folding, affecting diseases like cystic fibrosis where mutated proteins are misprocessed.
Vesicular Transport Models in the Golgi Apparatus
How materials move through the Golgi has been debated, with two main models. The vesicular transport model posits that proteins shuttle between cisternae via vesicles, maintaining static compartments. In contrast, the cisternal maturation model suggests cisternae mature progressively: a new cis cisterna forms from ER vesicles, advances while enzymes recycle backward, and becomes trans before fragmenting into vesicles.
Evidence supports maturation, especially in yeast and plants, where live imaging shows cisternae turnover. In animals, a hybrid may operate. Vesicles from the ER fuse at the ER-Golgi intermediate compartment (ERGIC) before entering the cis face. At the TGN, sorting yields constitutive (continuous secretion), regulated (signal-triggered, like neurotransmitter release), or lysosomal vesicles.
In polarized cells like epithelia, the Golgi directs proteins to apical or basolateral surfaces, crucial for tissue function. Plant cells use similar mechanisms but with actin-driven mobility for rapid response to pathogens.
Differences in Plant and Animal Golgi Apparatus
While sharing core functions, the Golgi in plants and animals differs significantly in structure and behavior. Animal Golgi forms a perinuclear ribbon of interconnected stacks, anchored by microtubules near the centrosome, facilitating centralized processing. Plant Golgi, however, consists of discrete, mobile dictyosomes scattered in the cytoplasm, moving along actin filaments at speeds up to 7 micrometers per second.
This mobility allows plants to reposition Golgi toward infection sites for quick immune molecule release. During mitosis, animal Golgi disassembles, while plant Golgi remains intact, supplying materials to the forming cell plate for division. Plants lack certain animal proteins like GRASPs for stacking, relying instead on tethers like golgins and COG complexes.
Functions diverge too: plant Golgi synthesizes cell wall polysaccharides like pectins and hemicelluloses, comprising up to 80% of its activity, absent in animals. The plant TGN doubles as an early endosome, merging secretory and endocytic paths more prominently.
Here’s a detailed comparison table:
| Aspect | Animal Golgi Apparatus | Plant Golgi Apparatus |
|---|---|---|
| Location and Organization | Perinuclear ribbon; stacks linked by tubules; near centrosome | Scattered dictyosomes; individual stacks; throughout cytoplasm |
| Mobility | Relatively immobile; microtubule-dependent | Highly mobile; actin filament-guided; speeds up to 7 μm/s |
| During Mitosis | Disassembles into fragments | Remains intact; aids cell plate formation |
| Key Proteins | Includes GRASPs, GM130, giantin for stacking and ribbon formation | Lacks GRASPs; relies on COG complex, SNAREs like SYP31/32, Arf GTPases |
| Functions | Protein/lipid modification; lysosome formation; hormone packaging | Same plus cell wall polysaccharide synthesis (pectins, hemicelluloses); immune response relocation |
| TGN Role | Sorting hub; less endosomal integration | Acts as early endosome; merges secretory/endocytic traffic |
| Stack Number | 10-20 per cell; 4-8 cisternae/stack | Hundreds of dictyosomes; variable cisternae |
| Response to Stress | Fragments under stress like viral infection | Relocates for defense; supports salt tolerance via proteins like beta-COP |
This table captures the adaptations suiting each kingdom’s needs, from animal tissue specialization to plant structural rigidity.
Presence and Variations in Different Organisms
The Golgi apparatus varies widely across eukaryotes. In mammals, it’s a single, centralized structure, but in insects like Drosophila, it’s dispersed similar to plants. Yeasts show diversity: Saccharomyces cerevisiae has unstacked, scattered cisternae, while Pichia pastoris features stacked ones. Protists can have elaborate setups with up to 60 cisternae, aiding complex secretion.
In fungi, the Golgi supports spore formation, and in algae, it aids pigment synthesis. Absent in prokaryotes like bacteria, its evolution likely coincided with eukaryotic complexity, enabling advanced secretion. Some parasites like Giardia have reduced Golgi, reflecting minimal needs.
Role of the Golgi Apparatus in Diseases
Defects in the Golgi apparatus contribute to various diseases, often through impaired processing or transport. Congenital disorders of glycosylation (CDGs) arise from mutations in Golgi enzymes, leading to faulty sugar attachment on proteins, causing developmental issues like intellectual disability and organ malformations. For example, CDG type II involves deficiencies in mannose trimming, affecting multiple systems.
In neurodegenerative conditions like Alzheimer’s, hyperphosphorylated tau proteins accumulate due to Golgi fragmentation, disrupting neuronal transport. Parkinson’s links to Golgi stress from alpha-synuclein buildup. Cancer cells often show altered Golgi structure, enhancing secretion of growth factors for tumor progression.
Autoimmune diseases may involve misprocessed antigens, and viral infections like HIV hijack the Golgi for envelope protein glycosylation. In plants, Golgi disruptions affect growth, pollen viability, and pathogen resistance, as seen in mutants with deformed stacks leading to sterility.
Understanding these links highlights the Golgi’s therapeutic potential, with research exploring drugs to stabilize its structure in diseases.
Evolutionary Insights into the Golgi Apparatus
From an evolutionary perspective, the Golgi apparatus likely emerged with the endomembrane system in early eukaryotes, enabling compartmentalized processing absent in prokaryotes. Its conservation across kingdoms suggests ancient origins, possibly from ER invaginations. Variations, like stacked vs. unstacked forms, reflect adaptations: centralized in animals for efficiency, dispersed in plants for flexibility.
Fossil evidence is scarce, but comparative genomics shows core proteins like COPI/II coats are shared, indicating divergence from a common ancestor. In protists, diverse forms bridge simple to complex, offering clues to its development.
Conclusion
In wrapping up, the Golgi apparatus emerges as an indispensable cellular hub, masterfully orchestrating the modification, sorting, and dispatch of vital molecules. Its stacked structure and dynamic functions underpin everything from daily cellular maintenance to specialized roles in development and defense. By exploring its history, variations, and implications in health, we gain a deeper appreciation for this microscopic marvel. Ongoing research continues to unveil its secrets, promising advances in medicine and biotechnology. Whether in a plant cell building walls or an animal neuron firing signals, the Golgi’s contributions are profoundly integral to life as we know it.
Read These Articles in Detail
- The Cell: The Fundamental Building Block of Life
- Prokaryotic Cells vs. Eukaryotic Cells: A Detailed Exploration
- Prokaryotic Cells: Definition, Anatomy, Functions, and Their Importance
- Eukaryotic Cells Explained: Definition, Structure, Functions, and Importance
- The Cell Membrane: Structure, Functions, and Evolutionary Significance
- The Cell Wall: Structure, Composition, Functions, and Evolutionary Significance
- Endomembrane System: A Detailed Exploration of the Cellular Machinery of Life
- Mitochondria: A Comprehensive Guide to Their Structure and Functions
- The Cell Envelope: The Protective Shield of Prokaryotic Cells
- Ribosomes: Definition, Structure, and Functions
- Endoplasmic Reticulum: Structure, Types, and Functions Explained
- The Golgi Apparatus: Structure, Functions, and Cellular Importance
- Cytoplasm and Nucleus: Structure, Functions, Differences, and Examples
- What Is Cell Signaling? Structure, Types, and Functions Explained
- Three Main Parts of a Nucleotide: Structure and Functions
- Enzymes: Definition, Structure, Classification, and Functions
- DNA: Structure, Function, Discovery, and Modern Applications
- DNA Replication: Steps, Process, and Genetic Importance
- Properties of DNA: Structure, Physical, and Chemical Characteristics
- DNA Packaging: How DNA Fits into the Nucleus of a Cell
- R-Factors and Plasmids: How Bacteria Develop Antibiotic Resistance
- Chromosomes: Structure, Types, Functions, and Role in Genetics
- Genetic Material: DNA, RNA, and Their Role in Heredity and Evolution
- Deoxyribose and Ribose: Structure and Function in DNA and RNA
- Differences Between Genes and DNA: Definitions, Structures, and Functions
- RNA: Definition, Structure, Types, Functions, and Its Role in Life
Frequently Asked Questions
FAQ 1: What is the Golgi apparatus and why is it important in cells?
The Golgi apparatus is a vital organelle found in most eukaryotic cells, acting like a cellular post office that processes, modifies, and packages proteins and lipids for various destinations. Discovered by Camillo Golgi in 1898, it consists of stacked, flattened membrane sacs called cisternae, which form the Golgi complex. These cisternae are organized near the cell’s nucleus, working closely with the endoplasmic reticulum (ER) to handle molecules essential for cellular function. Its importance lies in its ability to ensure proteins and lipids are correctly modified and delivered, supporting everything from hormone secretion to cell membrane maintenance.
This organelle plays a central role in the secretory pathway, taking raw materials from the ER and refining them for specific roles. For example, it adds sugar molecules to proteins to form glycoproteins, which are crucial for cell signaling and immune responses, like determining blood types. Without the Golgi apparatus, cells couldn’t effectively communicate, repair, or maintain their structures, leading to dysfunction. In specialized cells, such as those in the pancreas, it packages insulin for release, highlighting its critical role in health and disease management.
FAQ 2: How does the Golgi apparatus function in protein modification?
The Golgi apparatus is a key player in modifying proteins to ensure they function properly within or outside the cell. Proteins synthesized in the rough endoplasmic reticulum (ER) arrive at the Golgi’s cis face via vesicles. As they move through the stacked cisternae, enzymes in each compartment perform specific modifications. One major process is glycosylation, where sugar molecules are added or trimmed to create glycoproteins, enhancing protein stability or signaling capabilities.
For instance, in the cis Golgi, high-mannose sugars are trimmed from proteins. In the medial Golgi, sugars like N-acetylglucosamine are added, and in the trans Golgi, complex sugars like sialic acid finalize the process. These changes are vital for proteins to reach their correct destinations, such as the cell membrane or lysosomes. The Golgi also adds phosphate groups or sulfate groups in some cases, tailoring proteins for roles like enzyme activity in lysosomes. This sequential processing ensures proteins are ready for tasks like antibody production in immune cells, showcasing the organelle’s precision.
FAQ 3: What is the structure of the Golgi apparatus?
The Golgi apparatus has a highly organized structure designed for efficient molecular processing. It is primarily composed of flattened, disc-shaped sacs called cisternae, typically 0.5 to 1.0 micrometers in diameter, stacked parallel to form a Golgi stack. Most cells have four to eight cisternae per stack, though some single-celled organisms may have up to 60. These stacks are interconnected by tubules, and the entire structure is often located near the nucleus, close to the endoplasmic reticulum.
The Golgi has two distinct faces: the cis face, which receives vesicles from the ER, and the trans face, where processed molecules exit in vesicles. The trans Golgi network (TGN) acts as a sorting hub, directing vesicles to destinations like the plasma membrane or lysosomes. Small vesicles bud off the edges for transport, while larger vacuoles store materials. In animal cells, the Golgi forms a ribbon-like network anchored by microtubules, whereas in plants, it exists as scattered dictyosomes, highlighting its adaptability across organisms.
FAQ 4: How does the Golgi apparatus differ between plant and animal cells?
While the Golgi apparatus serves similar roles in plant and animal cells, its structure and behavior differ significantly. In animal cells, the Golgi forms a single, ribbon-like structure near the nucleus, with 10 to 20 interconnected stacks anchored by microtubules. This centralized setup is ideal for processing proteins and lipids in cells like neurons or glandular cells, where it packages neurotransmitters or hormones like insulin.
In contrast, plant cells have numerous dictyosomes, which are individual Golgi stacks scattered throughout the cytoplasm. These dictyosomes are mobile, moving along actin filaments at speeds up to 7 micrometers per second, allowing rapid repositioning for tasks like pathogen defense. Plant Golgi also synthesizes cell wall polysaccharides, such as pectins and hemicelluloses, which are critical for structural support and make up about 80% of its activity—a function absent in animals. During cell division, animal Golgi disassembles, but plant Golgi remains intact, aiding cell plate formation. These differences reflect the unique needs of each kingdom, from animal tissue specialization to plant structural rigidity.
FAQ 5: What role does the Golgi apparatus play in vesicular transport?
The Golgi apparatus is a central hub for vesicular transport, managing the movement of proteins and lipids through the cell. It receives vesicles from the endoplasmic reticulum (ER) at its cis face, where raw molecules enter for processing. As these molecules travel through the cisternae, they are modified and sorted, eventually reaching the trans Golgi network (TGN), where they are packaged into vesicles for delivery to destinations like the plasma membrane, lysosomes, or extracellular space.
Two models explain this transport: the vesicular transport model, where vesicles shuttle materials between static cisternae, and the cisternal maturation model, where cisternae mature from cis to trans, carrying cargo forward while enzymes recycle backward. Evidence, especially from plant and yeast studies, supports the maturation model, showing cisternae turnover. For example, in polarized cells like epithelial cells, the Golgi directs vesicles to specific surfaces (apical or basolateral), ensuring precise delivery. This process is critical for functions like neurotransmitter release in neurons or enzyme delivery to lysosomes.
FAQ 6: How does the Golgi apparatus contribute to disease when it malfunctions?
Malfunctions in the Golgi apparatus can lead to serious health issues due to its role in processing and transporting molecules. Congenital disorders of glycosylation (CDGs) result from defective Golgi enzymes, disrupting sugar attachment to proteins. This can cause developmental delays, intellectual disabilities, and organ issues, as seen in CDG type II, where mannose trimming fails. These disorders highlight the Golgi’s role in ensuring proper protein function.
In neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, Golgi fragmentation disrupts protein trafficking. For example, in Alzheimer’s, hyperphosphorylated tau proteins accumulate, impairing neuronal function. Cancer cells often show altered Golgi structures, increasing secretion of growth factors that fuel tumor growth. Viruses like HIV exploit the Golgi to glycosylate their envelope proteins, aiding infection. In plants, Golgi defects can reduce pollen viability or pathogen resistance, leading to sterility or susceptibility. These examples underscore the organelle’s critical role and its potential as a therapeutic target.
FAQ 7: What are the main functions of the Golgi apparatus in cells?
The Golgi apparatus performs a wide range of functions essential for cellular health and communication. Its primary role is protein modification, particularly glycosylation, where it adds or trims sugars to form glycoproteins, crucial for cell recognition and signaling. For instance, glycoproteins on red blood cells determine blood types. The Golgi also modifies lipids, producing glycolipids and sphingomyelin for cell membrane integrity and signaling.
Beyond modification, it packages molecules into vesicles for transport to destinations like the plasma membrane, lysosomes, or extracellular space. It contributes to lysosome formation by directing digestive enzymes, aiding waste management. In endocrine cells, it processes and packages hormones, such as insulin in pancreatic cells. In plants, it synthesizes cell wall polysaccharides and supports root hair formation for nutrient absorption. Additionally, it plays a role in membrane recycling and connective tissue matrix formation, showcasing its versatility across cell types.
FAQ 8: Why is the Golgi apparatus absent in some cells?
The Golgi apparatus is absent in certain cells where its functions are unnecessary or handled differently. For example, mature red blood cells in mammals lack a Golgi because they don’t secrete proteins or maintain complex organelles, focusing solely on oxygen transport. Similarly, mature sperm cells lack a Golgi, as their primary role is genetic delivery, not secretion, with most processing completed during development.
In prokaryotes like bacteria and blue-green algae, the absence of a Golgi reflects their simpler cellular structure, lacking the endomembrane system found in eukaryotes. These organisms rely on alternative mechanisms, like direct membrane secretion, for molecule transport. Some eukaryotic parasites, such as Giardia, have a reduced or absent Golgi due to minimal secretory needs, adapted to their parasitic lifestyle. These absences highlight how the Golgi’s presence is tied to the complexity of cellular tasks in eukaryotes.
FAQ 9: How does the Golgi apparatus vary across different organisms?
The Golgi apparatus shows remarkable variation across organisms, reflecting their unique biological needs. In mammals, it forms a centralized, ribbon-like structure near the nucleus, with 10 to 20 stacks connected by tubules, ideal for efficient processing in cells like neurons. In contrast, plant cells have numerous scattered dictyosomes, mobile units that support rapid responses, such as synthesizing cell wall polysaccharides or relocating during pathogen attacks.
Insects like Drosophila have dispersed Golgi similar to plants, while yeasts vary: Saccharomyces cerevisiae has unstacked cisternae, but Pichia pastoris features stacked ones. Single-celled protists may have up to 60 cisternae to handle complex secretion. In fungi, the Golgi supports spore formation, and in algae, it aids pigment synthesis. These variations underscore the organelle’s evolutionary adaptability, shaped by each organism’s environment and function, with core proteins conserved across eukaryotes.
FAQ 10: What is the evolutionary significance of the Golgi apparatus?
The Golgi apparatus likely emerged with the endomembrane system in early eukaryotes, marking a leap in cellular complexity. Its presence in most eukaryotes, but absence in prokaryotes, suggests it evolved to handle advanced secretion and processing needs. Scientists propose it originated from ER invaginations, creating compartments for specialized tasks like glycosylation and vesicle sorting, which enabled multicellular life.
Comparative genomics reveals conserved proteins, like COPI/II coats, across eukaryotes, indicating a common ancestor. Variations, such as stacked cisternae in mammals versus scattered dictyosomes in plants, reflect adaptations to specific lifestyles. In protists, diverse Golgi forms bridge simple to complex structures, offering clues to its development. This evolutionary flexibility allowed the Golgi to support diverse functions, from hormone secretion in animals to cell wall synthesis in plants, cementing its role in eukaryotic success.
FAQ 11: What is the role of the Golgi apparatus in cell secretion and exocytosis?
The Golgi apparatus plays a crucial part in the process of cell secretion, acting as the final checkpoint where proteins and lipids are prepared for release outside the cell. After proteins are synthesized in the endoplasmic reticulum, they travel to the Golgi in transport vesicles. Here, in the stacked cisternae, these molecules undergo modifications like glycosylation, where sugar chains are added to enhance their stability and functionality. This step is essential because it ensures the proteins are correctly folded and ready for their roles, whether as enzymes, hormones, or structural components. Once modified, the Golgi sorts these molecules into secretory vesicles at its trans face, which then move toward the plasma membrane.
Exocytosis, the mechanism by which these vesicles release their contents, is tightly regulated and often triggered by external signals, such as calcium influx in nerve cells releasing neurotransmitters. In cells that secrete large amounts of proteins, like those in the pancreas producing insulin, the Golgi forms specialized secretory granules that store these molecules until needed. This regulated exocytosis allows for controlled release, preventing wasteful or untimely secretion.
Interestingly, recent studies have shown that partial cargo release can occur through a process called kiss-and-run exocytosis, where the vesicle fuses temporarily with the membrane and then retracts, modulated by proteins like clathrin and dynamin. This fine-tuned system highlights how the Golgi not only packages but also influences the efficiency of secretion, impacting everything from hormone balance to immune responses.
Without the Golgi’s involvement, secretion would be chaotic, leading to cellular dysfunction. For example, in endocrine glands, the Golgi mediates hormone production by packaging them into vesicles, ensuring they are released in response to bodily needs. In chick embryos, it even synthesizes retinal pigments, demonstrating its role in developmental secretion. Overall, the Golgi’s integration into the secretory pathway underscores its importance as a dynamic hub, adapting to the cell’s demands and maintaining homeostasis through precise exocytosis.
FAQ 12: How does the Golgi apparatus interact with other cellular organelles?
The Golgi apparatus doesn’t operate in isolation; it forms a network of interactions with various organelles to ensure smooth cellular operations. Its closest partner is the endoplasmic reticulum (ER), from which it receives newly synthesized proteins and lipids via vesicles that fuse at the cis face. This handover is vital for the Golgi to perform its modifying functions, like adding carbohydrates or phosphates. Beyond the ER, the Golgi communicates with lysosomes by directing digestive enzymes to them, aiding in waste breakdown and recycling.
Key interactions include:
- With the centrosome: The Golgi often positions near the centrosome, using microtubules to maintain its structure and facilitate vesicle transport. This partnership helps in cell polarity, especially in migrating cells where the Golgi orients toward the direction of movement.
- With mitochondria: Physical contacts allow for calcium signaling and lipid exchange, supporting energy needs during high secretory activity. Disruptions here can lead to stress responses in diseases like neurodegeneration.
- With endosomes and lysosomes: The trans Golgi network sorts materials for lysosomal delivery, while recycling pathways bring components back, maintaining membrane balance.
- With the plasma membrane: Through exocytosis, Golgi-derived vesicles fuse directly, inserting proteins and lipids into the membrane for growth or signaling.
- With the nucleus: Though indirect, the Golgi’s perinuclear location in many cells suggests coordination during cell division, where it fragments and reassembles to distribute evenly to daughter cells.
These connections highlight the Golgi as a central player in the endomembrane system, where membrane contact sites enable non-vesicular communication, like lipid transfer or signaling cascades. In polarized cells, such as epithelial ones, these interactions ensure directional secretion, crucial for tissue function. Understanding these links has implications for diseases where Golgi fragmentation disrupts organelle harmony, leading to impaired trafficking.
FAQ 13: What techniques are used to study the Golgi apparatus?
Scientists employ a variety of methods to investigate the Golgi apparatus, ranging from classical staining to advanced imaging and biochemical assays. These techniques help visualize its structure, track its dynamics, and understand its functions in health and disease. Below is a detailed table summarizing key approaches, including their principles, advantages, and examples of applications.
| Technique | Principle | Advantages | Applications | Limitations |
|---|---|---|---|---|
| Electron Microscopy (EM) | Uses electron beams for high-resolution imaging of fixed cells, often with heavy metal stains like osmium. | Provides detailed ultrastructure of cisternae and vesicles. | Studying Golgi stacking in different cell types or during mitosis. | Static images; requires cell fixation, which may alter dynamics. |
| Fluorescence Microscopy | Labels Golgi proteins with fluorescent tags (e.g., GFP-fused markers) for live-cell imaging. | Allows real-time observation of Golgi movement and fragmentation. | Tracking vesicle trafficking or response to stress in living cells. | Potential phototoxicity; resolution limited compared to EM. |
| Golgi Staining (Silver Impregnation) | Original method by Camillo Golgi using silver nitrate to highlight the organelle in neurons. | Simple for light microscopy; reveals Golgi in tissue sections. | Historical studies on nervous system; still used for morphology in fixed samples. | Not specific; can produce artifacts mistaken for structures. |
| Density Gradient Centrifugation | Separates Golgi fractions based on buoyancy in sucrose gradients. | Isolates pure Golgi for biochemical analysis. | Measuring enzyme activity like glycosyltransferases. | Requires large cell samples; disrupts native interactions. |
| Vital Staining | Uses dyes like ceramides or sphingolipids that accumulate in Golgi membranes. | Non-invasive for live cells; highlights Golgi in real time. | Observing Golgi in protozoa or during development. | Dye specificity varies; may affect cell function. |
| Imaging Flow Cytometry | Combines flow cytometry with microscopy to analyze Golgi in thousands of cells. | High-throughput for quantitative data on fragmentation. | Screening drugs affecting Golgi in cancer cells. | Lower resolution than traditional microscopy. |
| Thin Section TEM | Prepares ultra-thin cell sections for transmission electron microscopy. | Reveals fine details of cisternae polarity and contacts. | Studying Golgi in muscle differentiation or disease states. | Labor-intensive preparation; not for live imaging. |
| Golgi Enrichment Kits | Uses spin-column methods to isolate Golgi from cell lysates. | Quick and requires less material than traditional centrifugation. | Proteomic studies of Golgi proteins in biotechnology. | Potential contamination from other organelles. |
These methods have evolved with technology, enabling deeper insights into the Golgi’s role. For instance, combining fluorescence with EM (correlative light-electron microscopy) bridges dynamic and structural views, advancing research in areas like innate immunity.
FAQ 14: How does the Golgi apparatus contribute to the immune system?
In the immune system, the Golgi apparatus emerges as more than just a processing center; it’s a dynamic platform that orchestrates innate immunity responses. Traditionally known for modifying and packaging proteins, recent discoveries reveal it acts as a signaling hub during infections. When pathogens invade, the Golgi detects stress through sensors like STING or MAVS, triggering pathways that produce interferons and cytokines to alert nearby cells. This innate response is crucial for containing viruses before adaptive immunity kicks in.
For antibody-secreting cells like plasma cells, the Golgi expands dramatically to handle the massive production of immunoglobulins. It glycosylates these antibodies, ensuring they recognize and bind to antigens effectively. In T-cells, Golgi health influences their ability to fight tumors; stress here can impair vesicle trafficking, reducing cytotoxic granule release and weakening anti-cancer activity. Disruptions in Golgi function link to autoimmune diseases, where misprocessed proteins might trigger erroneous immune attacks.
Moreover, viruses like HIV exploit the Golgi for their envelope glycosylation, highlighting its dual role in host defense and pathogen exploitation. In sepsis, Golgi-mediated secretion of inflammatory mediators can exacerbate or resolve the condition, depending on balance. These insights position the Golgi as a potential therapeutic target, where modulating its stress response could enhance immunotherapy or combat infections.
FAQ 15: What are the evolutionary origins of the Golgi apparatus?
The Golgi apparatus likely originated early in eukaryotic evolution, coinciding with the development of the endomembrane system that set eukaryotes apart from prokaryotes. Scientists believe it arose from invaginations of the ER or plasma membrane, creating compartments for specialized protein processing. This autogenous model suggests the Golgi evolved to handle increasing cellular complexity, enabling advanced secretion and compartmentalization absent in bacteria.
Evidence from comparative genomics supports this:
- Conservation of core proteins like COPI/II coats across eukaryotes indicates a common ancestor.
- In simple protists, Golgi forms bridge primitive to stacked structures, showing gradual sophistication.
- Loss of stacked cisternae in some parasites like Giardia points to reductive evolution, where minimal needs led to simplification.
- The organelle paralogy hypothesis proposes duplication and divergence of membrane components, explaining Golgi’s polarity.
- In animals, ribbon-like organization emerged later, possibly for efficient trafficking in multicellular tissues.
These origins tie to eukaryotic success, allowing diverse functions from lipid synthesis to glycosylation. Variations, like unstacked cisternae in some yeasts, reflect adaptations to environments, underscoring the Golgi’s flexible evolution.
FAQ 16: What is the significance of the Golgi apparatus in biotechnology and medicine?
The Golgi apparatus holds immense potential in biotechnology and medicine, serving as a target for drug development and a tool for protein engineering. Its role in processing therapeutic proteins makes it central to biomanufacturing, while dysfunction links to various diseases, opening avenues for treatments. Here’s a table outlining key applications and implications.
| Area | Description | Examples | Potential Benefits | Challenges |
|---|---|---|---|---|
| Biopharmaceutical Production | Golgi modifies recombinant proteins like monoclonal antibodies in cell factories. | Engineering CHO cells to enhance glycosylation for better drug efficacy. | Improved stability and targeting of therapeutics like insulin analogs. | Variability in glycosylation affecting batch consistency. |
| Cancer Therapy | Golgi stress in tumors promotes secretion of growth factors; inhibitors could halt this. | Targeting Golgi in breast cancer to reduce metastasis. | Biomarkers for immunotherapy selection; enhanced T-cell function. | Specificity to avoid harming healthy cells. |
| Neurodegenerative Diseases | Fragmented Golgi in Alzheimer’s impairs protein trafficking; stabilizers may help. | Drugs restoring Golgi integrity in Huntington’s disease models. | Slowing progression by reducing toxic aggregates. | Delivery across blood-brain barrier. |
| Viral Infections | Viruses hijack Golgi for assembly; antivirals disrupt this. | Inhibiting Golgi in HIV envelope processing. | Broad-spectrum antivirals for emerging pathogens. | Resistance development in viruses. |
| Congenital Disorders | Mutations in Golgi enzymes cause glycosylation defects; gene therapy corrects them. | Treating CDGs with enzyme replacements. | Personalized medicine for rare diseases. | Early diagnosis required for effectiveness. |
| Innate Immunity Enhancement | Golgi as a signaling platform; modulators boost antiviral responses. | Stimulating Golgi sensors like STING for vaccines. | Stronger immune therapies against infections. | Balancing to prevent autoimmunity. |
| Diagnostic Tools | Golgi morphology as a biomarker in biopsies. | Imaging Golgi fragmentation in Parkinson’s. | Non-invasive monitoring of disease progression. | Need for advanced imaging tech. |
These applications underscore the Golgi’s versatility, with ongoing research promising breakthroughs in targeted therapies.
FAQ 17: What are some common misconceptions about the Golgi apparatus?
Many people hold outdated or simplified views of the Golgi apparatus, often stemming from early textbooks or incomplete explanations. One widespread myth is that it’s a static structure, like a fixed factory; in reality, it’s highly dynamic, constantly reshaping through cisternal maturation and responding to cellular needs. Another misconception is that all protein traffic must pass through the Golgi—while most secretory proteins do, some bypass it directly from the ER to the plasma membrane.
Common misunderstandings include:
- Believing the Golgi is absent in prokaryotes due to simplicity, but it’s tied to eukaryotic evolution, not just complexity.
- Thinking it’s only for protein packaging; it also synthesizes lipids and acts in signaling, like in innate immunity.
- Assuming uniform structure across cells; variations like dictyosomes in plants or ribbons in animals reflect adaptations.
- Confusing it with artifacts from staining; early debates questioned its existence, but electron microscopy confirmed it.
- Overlooking its role in diseases; many think it’s minor, yet defects cause congenital disorders and contribute to cancer.
Clarifying these helps appreciate the Golgi’s true versatility and importance in cellular biology.
FAQ 18: How is the Golgi apparatus involved in cellular development and differentiation?
During cellular development, the Golgi apparatus undergoes remarkable changes to support differentiation, transitioning from a simple structure in stem cells to a more elaborate one in specialized tissues. In muscle cells, for instance, the Golgi reorganizes during fusion into myotubes, fragmenting and reassembling to handle increased secretion of contractile proteins. This adaptability ensures cells acquire their unique functions, like producing extracellular matrix in fibroblasts.
In keratinocytes, the Golgi aids in forming specialized lysosomes by distributing enzymes, crucial for skin barrier development. Disruptions can lead to improper differentiation, as seen in neurological disorders where Golgi defects impair dendrite growth in neurons. In embryonic stages, such as chick retinal pigment synthesis, the Golgi’s role in pigment packaging highlights its contribution to organ formation.
Overall, the Golgi’s involvement in trafficking growth factors and hormones drives differentiation signals, making it a key regulator in tissue morphogenesis and regeneration.
FAQ 19: How does the Golgi apparatus vary across different cell types?
The Golgi apparatus exhibits fascinating variations tailored to cellular demands, reflecting its adaptability. In secretory cells like plasma cells, it enlarges with more stacks to manage antibody production, while in neurons, it’s often polarized toward axons for neurotransmitter packaging. Below is a table comparing Golgi features in select cell types.
| Cell Type | Golgi Location | Structure | Key Functions | Unique Adaptations |
|---|---|---|---|---|
| Plasma Cells | Perinuclear, expanded stacks | Numerous cisternae (up to 20) | Antibody glycosylation and secretion | Hypertrophied for high-volume output |
| Neurons | Somatic, near nucleus | Ribbon-like, interconnected | Neurotransmitter packaging | Polarized for axonal transport |
| Epithelial Cells | Apical side | Stacked, polarized | Directional secretion to lumen | Oriented for tissue barrier maintenance |
| Fibroblasts | Scattered stacks | Variable cisternae | Matrix protein assembly | Flexible for wound healing responses |
| Sperm Cells (Mature) | Absent | N/A | N/A | Lost during maturation for streamlining |
| Pancreatic Beta Cells | Near ER, large | Multiple stacks | Insulin granule formation | Regulated for hormone storage |
| Plant Root Cells | Scattered dictyosomes | Small, mobile units | Root hair material synthesis | Actin-driven mobility for growth |
| Yeast Cells | Unstacked or scattered | Tubular compartments | Simple secretion | Adapted for rapid division |
These differences optimize the Golgi for specific roles, with size and organization varying by secretory load and polarity needs.
FAQ 20: What are the future directions in Golgi apparatus research?
Future research on the Golgi apparatus promises to uncover its untapped potential in disease treatment and cellular engineering. With advances in imaging and genomics, scientists aim to map Golgi dynamics in real time, revealing how stress affects T-cell efficacy in cancer immunotherapy. Targeting Golgi signaling could enhance innate immunity against viruses, while understanding its role in neurodegeneration might yield drugs to prevent fragmentation in Alzheimer’s.
Promising areas include:
- Developing Golgi-specific inhibitors for cancer, focusing on metastasis-driven secretion.
- Exploring Golgi as a biomarker for early disease detection, like in congenital glycosylation disorders.
- Investigating membrane contact sites with organelles for new lipid therapies.
- Using CRISPR to edit Golgi genes, studying evolutionary adaptations in model organisms.
- Integrating AI for predicting Golgi responses in drug screening.
These efforts could revolutionize medicine, turning the Golgi from a processing hub into a therapeutic powerhouse.
Acknowledgement
Examsmeta sincerely expresses its sincere gratitude to the numerous scientific resources that have contributed to the comprehensive understanding presented in the article “The Golgi Apparatus: Structure, Functions, and Cellular Importance.” The wealth of knowledge provided by these platforms has been instrumental in crafting a detailed and accurate exploration of the Golgi apparatus, its structure, functions, and significance in cellular biology. Their reliable and up-to-date information helped ensure the article’s depth and clarity, making complex concepts accessible to a broad audience.
Below are the key sources that informed this work:
- Nature (www.nature.com): Provided in-depth insights into the Golgi’s role in cellular processes and disease mechanisms.
- ScienceDirect (www.sciencedirect.com): Offered detailed studies on Golgi structure and vesicular transport models.
- PubMed (pubmed.ncbi.nlm.nih.gov): Supplied peer-reviewed research on the Golgi’s involvement in immunity and development.
- Cell Press (www.cell.com): Contributed cutting-edge findings on Golgi dynamics and biotechnology applications.
- Journal of Cell Biology (www.jcb.org): Shared critical data on Golgi evolution and organelle interactions.

