Nucleotides are fascinating molecules that form the very foundation of life as we know it. They serve as the building blocks for DNA and RNA, which carry the genetic instructions essential for the development, functioning, growth, and reproduction of all living organisms. Understanding the three main parts of a nucleotide not only helps us grasp how genetic information is stored and transmitted but also sheds light on processes like protein synthesis, energy transfer, and even modern biotechnologies.
In this in-depth article, we’ll explore every aspect of nucleotides, from their basic components to their broader implications in biology, drawing on established scientific insights to provide a clear and thorough explanation.
Table of Contents
Whether you’re a student diving into molecular biology for the first time or someone curious about the inner workings of cells, this guide breaks it down in simple terms. We’ll cover the structure, the key differences between nucleotides in DNA and RNA, their various functions, and much more. By the end, you’ll have a solid understanding of why these tiny molecules are so crucial to everything from inheritance to cellular energy.
What Exactly Are Nucleotides?
Nucleotides are organic molecules that act as the monomers, or single units, that link together to form long chains known as nucleic acids. The two primary nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA is like the master blueprint of an organism, storing genetic information in the nucleus of cells, while RNA acts as a messenger, helping to translate that information into proteins that perform vital tasks in the body.
Each nucleotide is made up of three distinct components: a sugar molecule, a nitrogenous base, and a phosphate group. These parts work in harmony to create the diversity and specificity needed for genetic coding. For instance, the sequence of nucleotides in a DNA strand determines traits like eye color or height, passed down from parents to offspring. Without nucleotides, life as we know it wouldn’t exist, as they enable the replication of genetic material during cell division and the response to environmental changes.
Nucleotides aren’t just limited to genetics; they play roles in energy storage and cellular signaling too. Think of adenosine triphosphate (ATP), a nucleotide derivative that’s often called the “energy currency” of cells. It powers everything from muscle contractions to nerve impulses. This versatility makes nucleotides indispensable in biological systems.
The Three Main Parts of a Nucleotide Explained
Let’s break down the three main parts of a nucleotide in detail. Each component has a unique structure and function, and together they form a molecule that’s both stable and adaptable.
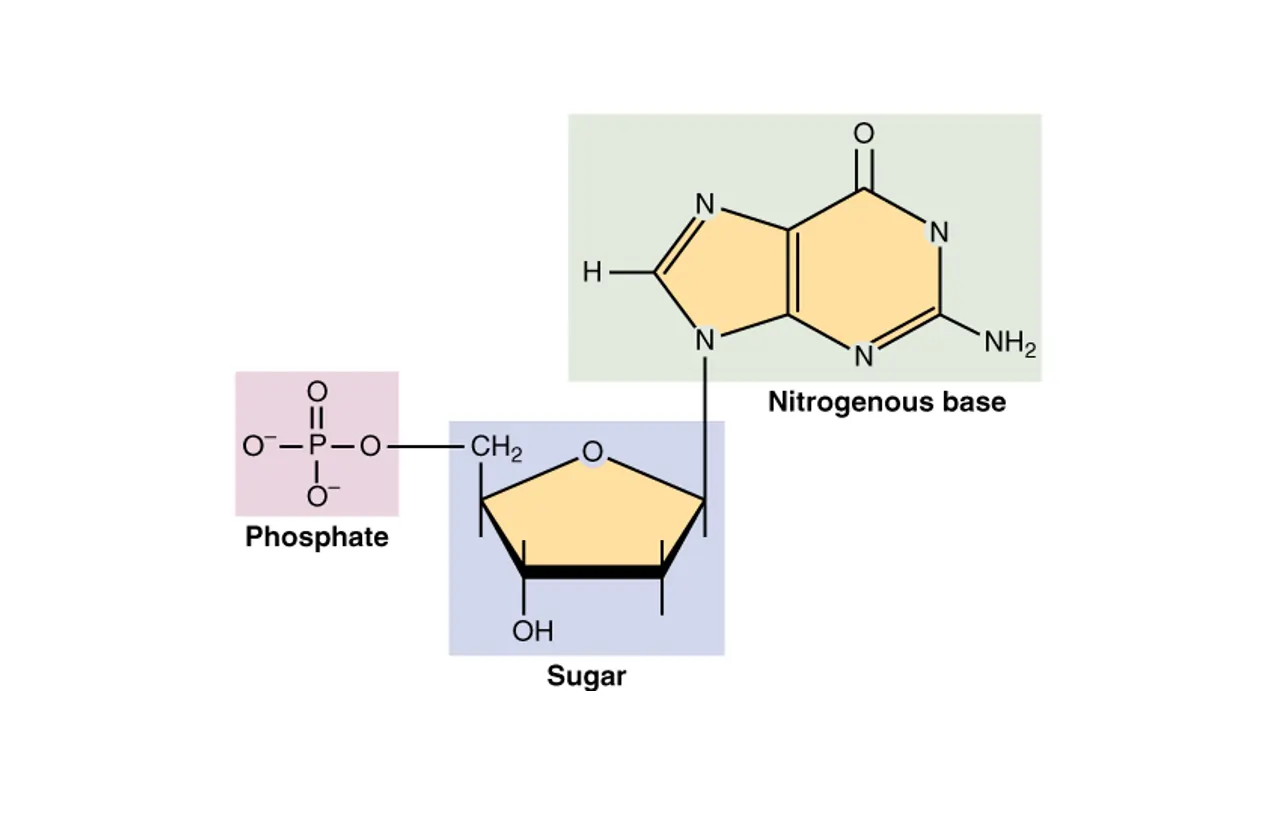
The Sugar Group: The Backbone Foundation
The first key part is the sugar group, which is a five-carbon sugar known as a pentose. In DNA, this sugar is called deoxyribose, while in RNA, it’s ribose. The difference might seem small, but it’s significant: deoxyribose lacks an oxygen atom at the 2′ carbon position (it has a hydrogen instead), which makes DNA more stable and less prone to breakdown compared to RNA.
The sugar molecule provides the structural framework for the nucleotide. Its five carbon atoms are numbered 1′ through 5′ (the prime symbol distinguishes them from the base’s numbering). The nitrogenous base attaches to the 1′ carbon, and the phosphate group links to the 5′ carbon. In a chain of nucleotides, the 3′ carbon of one sugar connects to the 5′ carbon of the next via a phosphodiester bond, creating the sugar-phosphate backbone that’s essential for the linear structure of DNA and RNA.
Why does this matter? The sugar’s configuration allows nucleic acids to form specific shapes. In DNA, the deoxyribose contributes to the iconic double helix, providing flexibility for twisting without breaking. In contrast, ribose in RNA makes it more reactive, which is why RNA is often single-stranded and involved in short-term tasks like protein synthesis.
For example, in human cells, deoxyribose in DNA ensures long-term storage of genetic data, while ribose in RNA allows for quick copying and use of that data. This distinction is why DNA can last for generations, but RNA molecules are constantly being made and broken down.
The Nitrogenous Base: The Information Carrier
Next up is the nitrogenous base, a ring-shaped molecule containing nitrogen that carries the genetic information. These bases are divided into two categories: purines and pyrimidines. Purines, like adenine (A) and guanine (G), have a double-ring structure made of nine atoms. Pyrimidines, such as cytosine (C), thymine (T), and uracil (U), have a single six-membered ring.
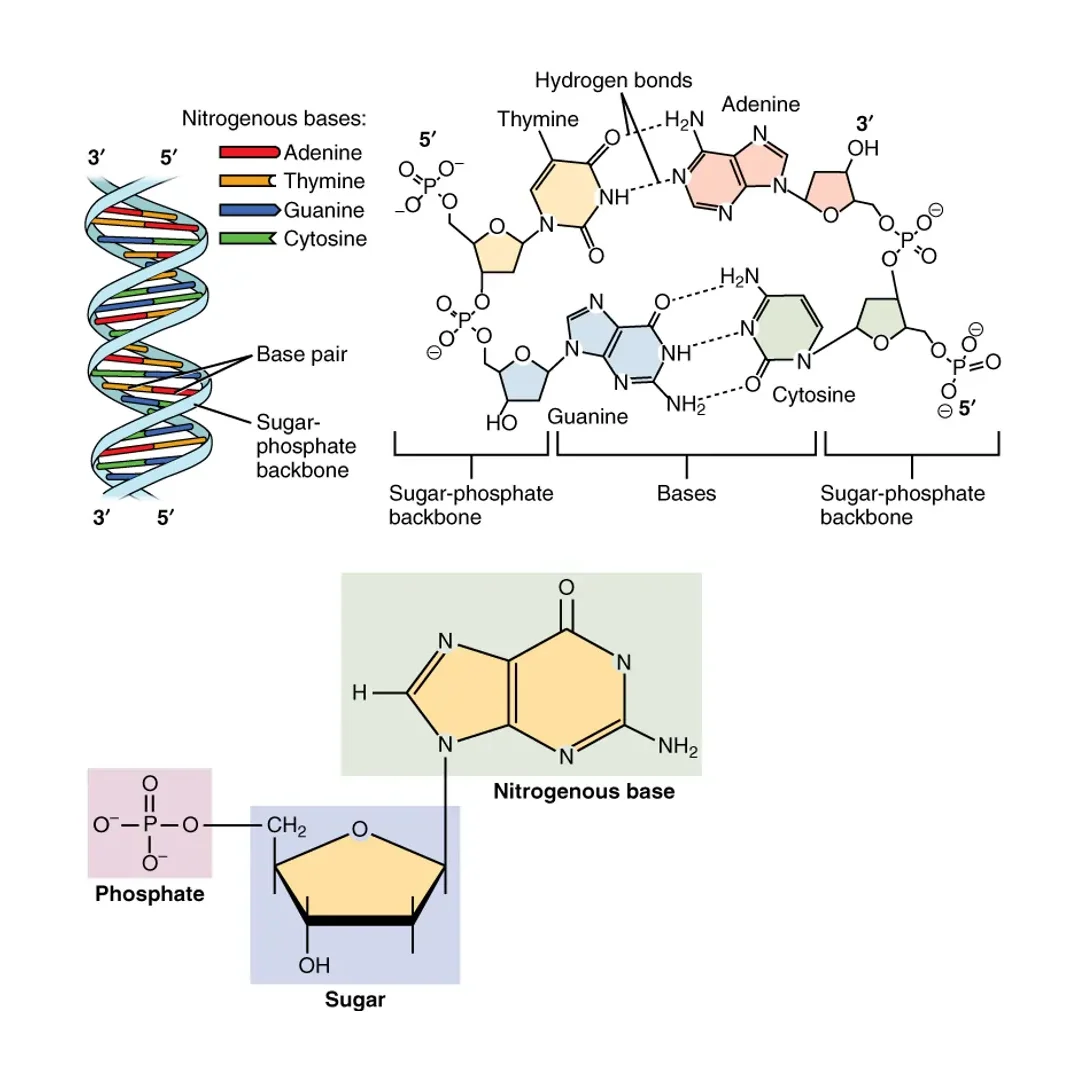
In DNA, the bases are A, T, C, and G. In RNA, T is replaced by U, so it’s A, U, C, and G. This base is attached to the 1′ carbon of the sugar and plays a critical role in base pairing, where complementary bases form hydrogen bonds: A pairs with T (or U in RNA) via two hydrogen bonds, and C pairs with G via three. This selectivity ensures accurate copying of genetic information during replication or transcription.
The arrangement of these bases along a nucleic acid chain forms the genetic code. For instance, a sequence like ATG in DNA codes for the amino acid methionine, the start signal for protein synthesis. Mutations, such as substituting one base for another, can lead to changes in proteins, sometimes causing diseases like sickle cell anemia, where a single base change alters hemoglobin structure.
Bases also influence the overall properties of nucleic acids. Purines are larger and more stable, while pyrimidines are smaller, allowing for precise fitting in the double helix. In RNA, uracil’s presence makes it easier for the molecule to fold into complex shapes, like the loops in transfer RNA (tRNA) that help deliver amino acids during protein building.
The Phosphate Group: The Linking and Charging Element
The third part is the phosphate group, a molecule consisting of phosphorus surrounded by oxygen atoms (PO4). It’s attached to the 5′ carbon of the sugar and gives nucleic acids their negative charge, making them acidic hence the name “nucleic acid.”
This group is crucial for linking nucleotides together through phosphodiester bonds. In a chain, the phosphate of one nucleotide bonds to the 3′ carbon of the next sugar, creating a strong, directional backbone that runs from 5′ to 3′. This directionality is vital for processes like DNA replication, where new strands are synthesized in the 5′ to 3′ direction.
The negative charge from phosphates allows nucleic acids to interact with positively charged proteins, like histones in chromosomes, which help package DNA tightly in the nucleus. In energy-related nucleotides like ATP, multiple phosphate groups (up to three) store high-energy bonds that release energy when broken, fueling cellular activities.
For example, during exercise, ATP breaks down to ADP (adenosine diphosphate) plus phosphate, providing the energy for muscle movement. In DNA and RNA, the phosphate’s hydrophilicity ensures these molecules stay dissolved in the watery environment of cells.
Detailed Structure of Nucleotides
A nucleotide’s structure can be visualized as a central sugar with a base sticking out like a flag and a phosphate attached like a handle. When nucleotides polymerize, they form polynucleotides: chains where the sugar-phosphate backbones alternate, and bases project inward for pairing.
In DNA, two antiparallel strands (one running 5′ to 3′, the other 3′ to 5′) twist into a double helix, stabilized by hydrogen bonds between bases and hydrophobic interactions. RNA is usually single-stranded but can fold back on itself, forming hairpins or loops for functions like catalysis in ribozymes.
Nucleosides are similar but lack the phosphate they’re just sugar plus base, like adenosine in caffeine or guanosine in some antiviral drugs. Adding one or more phosphates turns them into nucleotides.
Here’s a table summarizing the components:
| Component | Description | Attachment Point | Role in Structure |
|---|---|---|---|
| Sugar | Pentose (deoxyribose in DNA, ribose in RNA) | N/A | Forms backbone with phosphate |
| Nitrogenous Base | Purine (A, G) or Pyrimidine (C, T/U) | 1′ carbon | Carries genetic code, base pairing |
| Phosphate Group | PO4, provides negative charge | 5′ carbon | Links nucleotides via bonds |
Key Differences Between DNA and RNA Nucleotides
While both DNA and RNA nucleotides share the same basic three parts, subtle differences tailor them to their roles.
- Sugar: DNA’s deoxyribose lacks an OH group at 2′, making it more resistant to hydrolysis and suitable for long-term storage. RNA’s ribose has that OH, increasing reactivity for transient roles.
- Bases: DNA uses thymine, which has a methyl group for added stability against mutations. RNA’s uracil is simpler and cheaper to produce, fitting its disposable nature.
- Structure and Function: DNA is double-stranded for protection, while RNA is single-stranded for versatility in mRNA (messenger), tRNA (transfer), and rRNA (ribosomal).
- Location: DNA resides in the nucleus (or mitochondria), RNA is found in nucleus, cytoplasm, and ribosomes.
A comparison table:
| Feature | DNA Nucleotides | RNA Nucleotides |
|---|---|---|
| Sugar | Deoxyribose | Ribose |
| Bases | A, T, C, G | A, U, C, G |
| Strand Structure | Double helix | Mostly single-stranded |
| Primary Function | Genetic storage and inheritance | Protein synthesis and gene regulation |
| Stability | High, due to deoxyribose and double strand | Lower, more reactive |
| Examples | Chromosomal DNA, mitochondrial DNA | mRNA, tRNA, rRNA, viral RNA |
These differences ensure DNA safeguards the genome, while RNA actively uses it.
Functions of Nucleotides
Nucleotides do far more than just form DNA and RNA. Here’s a breakdown of their diverse roles:
- Genetic Information Storage and Transmission: In DNA, nucleotide sequences encode genes. During replication, enzymes like DNA polymerase add complementary nucleotides, ensuring accurate copying.
- Protein Synthesis: RNA nucleotides are key. Transcription copies DNA into mRNA nucleotides, which ribosomes read in triplets (codons) to build proteins via tRNA bringing amino acids.
- Energy Transfer: ATP, with three phosphates, stores energy from food breakdown and releases it for work. ADP recycles back to ATP in mitochondria.
- Coenzymes and Cofactors: NAD (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide) carry electrons in metabolism, aiding reactions like cellular respiration.
- Cell Signaling: Cyclic AMP (cAMP), a modified nucleotide, acts as a second messenger, amplifying hormone signals to trigger responses like glycogen breakdown.
- Enzymatic Roles: Some RNA molecules, called ribozymes, use nucleotides to catalyze reactions, like splicing introns from mRNA.
- Structural Components: In viruses, RNA nucleotides form the genome, as in COVID-19’s single-stranded RNA.
Examples abound: In bacteria, nucleotide sequences in plasmids confer antibiotic resistance. In humans, faulty nucleotide repair leads to cancers from mutations.
A functions table:
| Function | Description | Example Molecule or Process |
|---|---|---|
| Building Blocks | Form DNA/RNA chains | Polymerization in replication |
| Energy Carrier | Store and release energy | ATP in muscle contraction |
| Coenzyme | Assist enzymes in reactions | NAD in glycolysis |
| Signaling | Transmit intracellular messages | cAMP in hormone response |
| Genetic Coding | Sequence determines traits | Codons in protein synthesis |
| Catalytic | RNA acts as enzyme | Ribozymes in splicing |
Historical Insights and Discovery of Nucleotides
The story of nucleotides begins in the 19th century. In 1869, Swiss scientist Friedrich Miescher isolated a substance he called “nuclein” from white blood cells, later identified as nucleic acids. By the early 1900s, researchers like Phoebus Levene identified the three components: sugar, base, and phosphate, coining the term “nucleotide.”
Watson and Crick’s 1953 double helix model, built on X-ray data from Rosalind Franklin, revealed how nucleotides pair and twist. This paved the way for understanding replication and the genetic code, cracked in the 1960s.
Today, nucleotide knowledge drives fields like forensics (DNA fingerprinting) and medicine (nucleotide analogs in chemotherapy).
Nucleotides in Biotechnology and Medicine
Modern applications highlight nucleotides’ importance. In PCR (polymerase chain reaction), nucleotides are added to amplify DNA for testing. mRNA vaccines, like those for COVID-19, use synthetic RNA nucleotides to instruct cells to produce viral proteins, training the immune system.
In gene therapy, modified nucleotides correct defective genes, treating conditions like spinal muscular atrophy. Nucleotide-based drugs, such as antisense oligonucleotides, silence harmful genes in diseases like Duchenne muscular dystrophy.
Challenges include ensuring stability RNA nucleotides degrade quickly, so vaccines use lipid nanoparticles for protection.
Common Misconceptions and Advanced Concepts
One myth is that all nucleotides are identical; in reality, their bases create endless variety, with human DNA having about 3 billion nucleotide pairs. Another is confusing nucleosides with nucleotides; remember, nucleosides lack phosphate.
Advanced ideas include nucleotide modifications, like methylation in epigenetics, which regulates gene expression without changing the sequence. In viruses, unusual nucleotides help evade immune detection.
Conclusion
In wrapping up, the three main parts of a nucleotide the sugar, nitrogenous base, and phosphate group form a remarkably versatile molecule central to life. From storing genetic blueprints in DNA to powering cells with ATP, nucleotides underpin countless biological processes. Their differences in DNA and RNA allow for specialized roles, ensuring efficiency in inheritance and daily cellular functions.
As research advances, our understanding of nucleotides continues to grow, opening doors to innovations in health and technology. Whether exploring your own genome or marveling at life’s complexity, remember: it all starts with these three simple yet profound parts.
Read These Articles in Detail
- The Cell: The Fundamental Building Block of Life
- Prokaryotic Cells vs. Eukaryotic Cells: A Detailed Exploration
- Prokaryotic Cells: Definition, Anatomy, Functions, and Their Importance
- Eukaryotic Cells Explained: Definition, Structure, Functions, and Importance
- The Cell Membrane: Structure, Functions, and Evolutionary Significance
- The Cell Wall: Structure, Composition, Functions, and Evolutionary Significance
- Endomembrane System: A Detailed Exploration of the Cellular Machinery of Life
- Mitochondria: A Comprehensive Guide to Their Structure and Functions
- The Cell Envelope: The Protective Shield of Prokaryotic Cells
- Ribosomes: Definition, Structure, and Functions
- Endoplasmic Reticulum: Structure, Types, and Functions Explained
- The Golgi Apparatus: Structure, Functions, and Cellular Importance
- Cytoplasm and Nucleus: Structure, Functions, Differences, and Examples
- What Is Cell Signaling? Structure, Types, and Functions Explained
- Three Main Parts of a Nucleotide: Structure and Functions
- Enzymes: Definition, Structure, Classification, and Functions
- DNA: Structure, Function, Discovery, and Modern Applications
- DNA Replication: Steps, Process, and Genetic Importance
- Properties of DNA: Structure, Physical, and Chemical Characteristics
- DNA Packaging: How DNA Fits into the Nucleus of a Cell
- R-Factors and Plasmids: How Bacteria Develop Antibiotic Resistance
- Chromosomes: Structure, Types, Functions, and Role in Genetics
- Genetic Material: DNA, RNA, and Their Role in Heredity and Evolution
- Deoxyribose and Ribose: Structure and Function in DNA and RNA
- Differences Between Genes and DNA: Definitions, Structures, and Functions
- RNA: Definition, Structure, Types, Functions, and Its Role in Life
Frequently Asked Questions
FAQ 1: What Are the Three Main Parts of a Nucleotide?
Nucleotides are the fundamental building blocks of DNA and RNA, the molecules responsible for storing and transmitting genetic information in all living organisms. Each nucleotide is composed of three essential parts: a sugar molecule, a nitrogenous base, and a phosphate group. These components work together to create the structure of nucleic acids, enabling processes like genetic coding, protein synthesis, and even energy transfer in cells. Understanding these parts is key to grasping how life’s instructions are stored and executed at a molecular level.
The sugar molecule is a five-carbon sugar, either deoxyribose in DNA or ribose in RNA. Deoxyribose lacks an oxygen atom at the 2′ carbon, making DNA more stable for long-term genetic storage. Ribose, with its extra oxygen, makes RNA more reactive, suitable for temporary roles like carrying genetic messages. The sugar forms the backbone of the nucleotide, providing a scaffold that connects with other components.
The nitrogenous base carries the genetic information. In DNA, the bases are adenine (A), thymine (T), cytosine (C), and guanine (G), while RNA replaces thymine with uracil (U). These bases pair specifically (A with T/U, C with G) through hydrogen bonds, ensuring accurate replication and transcription. The phosphate group, made of phosphorus and oxygen, links nucleotides together via phosphodiester bonds, forming the sugar-phosphate backbone of DNA and RNA strands. This group also gives nucleic acids their negative charge, aiding interactions with proteins and other molecules. Together, these three parts make nucleotides versatile molecules critical for life.
FAQ 2: Why Are Nucleotides Important in DNA and RNA?
Nucleotides are the core units that make up DNA and RNA, which are essential for storing, transmitting, and expressing genetic information. Without nucleotides, the processes that allow cells to function, replicate, and pass traits from one generation to the next wouldn’t exist. Their importance lies in their ability to form the genetic code, act as structural components, and support various cellular functions.
In DNA, nucleotides form long chains that create the famous double helix structure. The sequence of nitrogenous bases (adenine, thymine, cytosine, guanine) encodes genetic instructions, like a blueprint for building proteins or determining traits like eye color. During cell division, nucleotides ensure accurate replication of this code, maintaining genetic continuity. For example, when a cell divides, enzymes like DNA polymerase use nucleotides to copy the entire genome.
In RNA, nucleotides play a dynamic role in protein synthesis. Messenger RNA (mRNA) carries the genetic code from DNA to ribosomes, where transfer RNA (tRNA) and ribosomal RNA (rRNA) use nucleotides to assemble amino acids into proteins. Beyond genetics, nucleotides like ATP (adenosine triphosphate) serve as energy carriers, powering processes like muscle movement. Nucleotides also act in cell signaling, with molecules like cAMP relaying messages inside cells. Their diverse roles make them indispensable to life.
FAQ 3: How Do the Sugar Molecules Differ in DNA and RNA Nucleotides?
The sugar molecule in a nucleotide is a critical component, and it differs between DNA and RNA, affecting their stability and function. In DNA, the sugar is deoxyribose, while in RNA, it’s ribose. This small chemical difference has a big impact on how these molecules work in cells, tailoring them to their specific roles in genetic storage and expression.
Deoxyribose in DNA lacks an oxygen atom at the 2′ carbon, replaced by a hydrogen atom. This makes DNA more chemically stable, ideal for long-term storage of genetic information in the nucleus. For instance, DNA in human cells can remain intact for years, faithfully preserving the genetic code across generations. The stability of deoxyribose helps DNA resist breakdown in the cellular environment.
Ribose in RNA, on the other hand, has a hydroxyl (OH) group at the 2′ carbon, making it more reactive. This reactivity suits RNA’s temporary roles, like carrying genetic instructions during protein synthesis. RNA molecules, such as mRNA, are often quickly made and broken down, allowing cells to respond rapidly to changes. For example, during viral infections, RNA’s reactivity allows quick production of viral proteins. This difference in sugars ensures DNA and RNA are specialized for their distinct biological tasks.
FAQ 4: What Role Do Nitrogenous Bases Play in Nucleotides?
Nitrogenous bases are the information-carrying components of nucleotides, acting like the letters of the genetic alphabet in DNA and RNA. These bases adenine, thymine (or uracil in RNA), cytosine, and guanine determine the sequence that encodes genetic instructions, making them crucial for processes like inheritance, protein synthesis, and cellular regulation.
There are two types of bases: purines (adenine and guanine), which have a double-ring structure, and pyrimidines (cytosine, thymine, uracil), which have a single-ring structure. In DNA, adenine pairs with thymine via two hydrogen bonds, and guanine pairs with cytosine via three, creating the stable double helix. In RNA, uracil replaces thymine, pairing with adenine. This base pairing ensures accurate copying during DNA replication and transcription, where DNA’s code is transferred to RNA.
The sequence of bases forms the genetic code. For example, a sequence like AUG in RNA signals the start of protein synthesis, coding for the amino acid methionine. Errors in base sequences, known as mutations, can lead to diseases like cystic fibrosis, where a single base change disrupts a protein’s function. Beyond genetics, bases in modified nucleotides, like those in tRNA, help fold RNA into shapes needed for its tasks, highlighting their versatility.
FAQ 5: How Does the Phosphate Group Contribute to Nucleotide Function?
The phosphate group in a nucleotide, made of phosphorus and oxygen atoms, is a small but mighty component that plays several critical roles in the structure and function of DNA, RNA, and other nucleotide-based molecules. Its unique properties make it essential for linking nucleotides and enabling cellular processes.
In nucleic acids, the phosphate group forms phosphodiester bonds, connecting the 5′ carbon of one nucleotide’s sugar to the 3′ carbon of the next. This creates the sugar-phosphate backbone, a strong, directional framework that gives DNA its double helix shape and RNA its flexible structure. The directionality (5′ to 3′) is vital for processes like DNA replication and transcription, where enzymes read or build strands in a specific order.
The phosphate group also gives nucleic acids their negative charge, making them acidic hence the term nucleic acid. This charge allows DNA to interact with positively charged proteins, like histones, which package DNA into chromosomes. In energy-carrying nucleotides like ATP, multiple phosphate groups store high-energy bonds that release energy for cellular work, such as nerve signaling or muscle contraction. For example, when ATP loses a phosphate to become ADP, it powers processes like ion transport across cell membranes.
FAQ 6: What Is the Difference Between a Nucleotide and a Nucleoside?
The terms nucleotide and nucleoside are often confused, but they refer to distinct molecules with different roles. A nucleotide is the complete unit that forms DNA and RNA, consisting of a sugar, a nitrogenous base, and a phosphate group. A nucleoside, however, lacks the phosphate group, containing only the sugar and base. This difference affects their functions in biological systems.
Nucleotides are the building blocks of nucleic acids. The phosphate group enables them to link together via phosphodiester bonds, forming long chains that store genetic information or carry out protein synthesis. For example, in DNA, nucleotides create the double helix, while in ATP, the phosphates store energy for cellular activities like muscle movement.
Nucleosides, without the phosphate, can’t form these chains but have other roles. For instance, adenosine, a nucleoside, is found in molecules like cAMP, which acts in cell signaling, or in drugs like adenosine used to treat heart rhythm disorders. In antiviral therapies, nucleoside analogs like acyclovir mimic nucleosides to disrupt viral replication. The absence of the phosphate group makes nucleosides smaller and suited for specific biochemical tasks, while nucleotides are the structural and functional backbone of nucleic acids.
FAQ 7: How Do Nucleotides Form the Genetic Code?
The genetic code is the set of instructions encoded in the sequence of nucleotides in DNA and RNA, determining how cells build proteins and express traits. The nitrogenous bases in nucleotides adenine, thymine (or uracil), cytosine, and guanine act like letters in a code, and their specific order creates the blueprint for life.
In DNA, nucleotides are arranged in long chains, with the sequence of bases (e.g., ATGGCC) specifying genes. These sequences are read in groups of three, called codons, during transcription, where DNA is copied into mRNA. Each codon corresponds to a specific amino acid or a stop signal in protein synthesis. For example, the codon AUG codes for methionine and signals the start of a protein chain. This process, called translation, occurs at ribosomes, where tRNA matches codons to amino acids, assembling proteins.
The precision of base pairing ensures the genetic code is accurately copied and expressed. Errors in nucleotide sequences, known as mutations, can alter the code, potentially causing disorders like sickle cell anemia, where a single base change affects hemoglobin. The genetic code’s universality across species highlights nucleotides’ critical role in life’s diversity and continuity.
FAQ 8: What Are the Functions of Nucleotides Beyond DNA and RNA?
While nucleotides are best known as the building blocks of DNA and RNA, their roles extend far beyond genetic material. These versatile molecules are involved in energy transfer, cell signaling, enzymatic reactions, and even structural functions, making them essential for cellular life.
One major role is energy transfer. Adenosine triphosphate (ATP), a nucleotide with three phosphate groups, is the cell’s primary energy carrier. When a phosphate bond breaks, it releases energy for processes like muscle contraction or nerve impulse transmission. For example, during exercise, ATP powers muscle fibers by converting to ADP. Nucleotides like NAD and FAD act as coenzymes, carrying electrons in metabolic pathways like cellular respiration, which produces energy from food.
In cell signaling, cyclic AMP (cAMP), derived from a nucleotide, acts as a second messenger, relaying signals from hormones to trigger responses like glucose release. Nucleotides also contribute to enzymatic functions; ribozymes, RNA molecules with catalytic activity, use nucleotides to perform tasks like RNA splicing. In biotechnology, synthetic nucleotides are used in drugs and vaccines, showcasing their broad impact beyond nucleic acids.
FAQ 9: How Do Nucleotides Contribute to Biotechnology and Medicine?
Nucleotides are at the heart of many breakthroughs in biotechnology and medicine, from genetic testing to life-saving therapies. Their roles in DNA and RNA make them critical for understanding and manipulating genetic information, while their derivatives power innovative treatments and diagnostics.
In biotechnology, nucleotides are essential for techniques like PCR (polymerase chain reaction), where they’re used to amplify DNA for applications like forensic analysis or paternity testing. mRNA vaccines, such as those for COVID-19, rely on synthetic RNA nucleotides to instruct cells to produce viral proteins, triggering an immune response. These vaccines use modified nucleotides to enhance stability and reduce immune overreactions, showcasing nucleotides’ adaptability.
In medicine, nucleotide analogs are used in antiviral and cancer therapies. Drugs like azidothymidine (AZT) for HIV or fluorouracil for cancer mimic nucleotides to interfere with viral or tumor cell replication. Gene therapy uses nucleotides to deliver corrected genes, treating disorders like cystic fibrosis. Nucleotides’ negative charge and base specificity make them ideal for designing precise treatments, revolutionizing healthcare.
FAQ 10: How Were Nucleotides Discovered, and Why Does Their History Matter?
The discovery of nucleotides marked a turning point in understanding life’s molecular basis. In 1869, Friedrich Miescher isolated a substance he called “nuclein” from white blood cells, later identified as nucleic acids containing nucleotides. This laid the groundwork for modern genetics, showing that molecules could carry hereditary information.
In the early 20th century, scientists like Phoebus Levene defined nucleotides as having a sugar, nitrogenous base, and phosphate group. The 1953 discovery of DNA’s double helix by Watson and Crick, using data from Rosalind Franklin, revealed how nucleotides pair and form DNA’s structure. This breakthrough explained replication and the genetic code, solved in the 1960s, showing how nucleotide sequences encode proteins.
This history matters because it underpins fields like molecular biology and medicine. Understanding nucleotides enabled technologies like DNA sequencing, which maps genomes, and CRISPR, which edits genes using nucleotide-based guides. It also informs our fight against diseases, as seen in nucleotide-based therapies for cancer or genetic disorders. Knowing this history highlights nucleotides’ transformative impact on science and human health.
FAQ 11: How Are Nucleotides Synthesized in Cells?
Nucleotides are essential molecules in all living cells, and their synthesis occurs through two primary pathways: the de novo pathway and the salvage pathway. The de novo pathway involves building nucleotides from scratch using simple precursor molecules like amino acids, carbon dioxide, and ribose sugars derived from the pentose phosphate pathway. This process is energy-intensive and crucial during periods of rapid cell growth, such as in developing tissues or cancer cells, where high demands for DNA and RNA require fresh nucleotide production.
For instance, the de novo synthesis of purine nucleotides starts with ribose-5-phosphate and involves multiple enzymatic steps to form inosine monophosphate (IMP), which then branches to create adenine and guanine nucleotides. Similarly, pyrimidine synthesis begins with carbamoyl phosphate and leads to uridine monophosphate (UMP), the precursor for cytosine, thymine, and uracil nucleotides.
In contrast, the salvage pathway recycles pre-existing bases and nucleosides from degraded nucleic acids, which is more energy-efficient and predominates in mature cells or under normal conditions. Enzymes like hypoxanthine-guanine phosphoribosyltransferase (HGPRT) play a key role here, attaching phosphoribosyl pyrophosphate (PRPP) to free bases to reform nucleotides. This pathway helps conserve resources and is linked to central metabolic routes like glycolysis and the citric acid cycle, ensuring a steady nucleotide supply for cellular functions.
The choice between these pathways depends on cellular needs and environmental factors. In proliferating cells, de novo synthesis ramps up to support DNA replication, while salvage dominates in non-dividing cells to maintain nucleotide pools. Disruptions in these pathways can lead to metabolic imbalances, highlighting their importance in health and disease. Overall, nucleotide synthesis is a finely tuned process integral to life, balancing efficiency with demand.
FAQ 12: What Genetic Disorders Are Associated with Nucleotide Metabolism?
Genetic disorders related to nucleotide metabolism arise from mutations in enzymes involved in purine or pyrimidine pathways, leading to imbalances that affect various body systems. These conditions are rare but can have severe impacts, including neurological issues, immune deficiencies, and metabolic problems. For example, Lesch-Nyhan syndrome is an X-linked disorder caused by a deficiency in HGPRT, resulting in purine overproduction, hyperuricemia, gout, and neurological symptoms like self-mutilation and developmental delays. This highlights how faulty salvage pathways can lead to toxic buildup of uric acid.
Another group involves purine nucleotide synthesis disorders, such as adenosine deaminase (ADA) deficiency, which impairs immune function and causes severe combined immunodeficiency (SCID), making affected individuals highly susceptible to infections. On the pyrimidine side, hereditary orotic aciduria stems from uridine monophosphate synthase deficiency, leading to megaloblastic anemia, growth retardation, and excess orotic acid excretion. These disorders often present in childhood with multisystem symptoms, underscoring the broad role of nucleotides beyond genetics.
Diagnosis typically involves biochemical tests for metabolite levels, and treatments may include dietary restrictions, enzyme replacement, or gene therapy. Understanding these disorders emphasizes the critical balance in nucleotide metabolism for overall health.
FAQ 13: What Is the Broader Role of Nucleotides in Cellular Metabolism?
Nucleotides extend far beyond their structural roles in DNA and RNA, serving as key players in cellular metabolism by providing energy, regulating pathways, and facilitating signaling. In energy metabolism, adenosine triphosphate (ATP) acts as the primary currency, fueling reactions like muscle contraction and active transport across membranes. Other nucleotides like GTP contribute to protein synthesis and signal transduction.
In redox reactions, coenzymes such as NAD+ and FAD, derived from nucleotides, carry electrons during cellular respiration, linking glycolysis to the electron transport chain for ATP production. Nucleotides also influence metabolic regulation; for instance, AMP-activated protein kinase (AMPK) senses low energy states and adjusts metabolism to restore balance.
| Aspect of Metabolism | Role of Nucleotides | Examples |
|---|---|---|
| Energy Provision | Supply chemical energy for cellular processes | ATP in glycolysis and citric acid cycle |
| Redox Reactions | Act as electron carriers | NAD+ in oxidative phosphorylation |
| Signaling and Regulation | Modulate enzyme activity and pathways | cAMP as second messenger in hormone responses |
| Biosynthesis Support | Precursors for macromolecules | GTP in microtubule assembly |
| Stress Response | Help manage oxidative stress | Nucleotide pools in cancer cell adaptation |
This table illustrates how nucleotides integrate into metabolic networks, making them indispensable for cellular homeostasis.
FAQ 14: Why Are Nucleotides Important in Nutrition and Diet?
Incorporating nucleotides into the diet can support various health aspects, particularly during growth, stress, or recovery periods. While the body can synthesize nucleotides, dietary sources become crucial when demands exceed production, such as in infants, athletes, or those with weakened immunity. Breast milk is rich in nucleotides, promoting infant gut development and immune maturation, which is why many infant formulas are supplemented with them to mimic these benefits.
Dietary nucleotides enhance intestinal health by fostering beneficial gut bacteria and aiding mucosal repair, potentially reducing infection risks. In adults, they may boost immune responses during challenges like surgery or malnutrition, as studies show nucleotide-supplemented nutrition improves recovery and resistance to pathogens.
Foods like organ meats, seafood, legumes, and yeast extracts provide natural nucleotides, contributing to overall well-being. However, excessive intake isn’t necessary for healthy individuals, as the body efficiently recycles them.
FAQ 15: What Are Synthetic Nucleotides and Their Medical Applications?
Synthetic nucleotides are lab-engineered versions of natural nucleotides, designed to mimic or modify biological processes for therapeutic purposes. These include analogs like azidothymidine (AZT) for HIV treatment, which incorporate into viral DNA to halt replication. In cancer therapy, drugs such as fluorouracil disrupt pyrimidine synthesis in rapidly dividing cells, slowing tumor growth.
Advances in nucleic acid therapeutics use synthetic oligonucleotides for gene silencing, as in antisense oligonucleotides (ASOs) that target faulty mRNA in genetic disorders like spinal muscular atrophy. mRNA vaccines, employing modified nucleotides for stability, revolutionized responses to pandemics by instructing cells to produce antigens.
- Antiviral Therapies: Synthetic nucleotides block viral enzymes, reducing infection spread.
- Cancer Treatments: They inhibit nucleotide metabolism in tumors, enhancing chemotherapy efficacy.
- Gene Editing: Tools like CRISPR use nucleotide guides for precise DNA modifications.
- Diagnostic Tools: Aptamers, synthetic nucleotide sequences, detect biomarkers for early disease identification.
These applications showcase synthetic nucleotides’ potential in personalized medicine.
FAQ 16: How Do Nucleotides Relate to the Origin of Life and Evolution?
Nucleotides likely played a pivotal role in the origin of life, forming the basis of the RNA world hypothesis, where RNA molecules served as both genetic material and catalysts before DNA and proteins evolved. In prebiotic conditions, simple nucleotides could have self-assembled in warm ponds or hydrothermal vents, polymerizing into RNA strands capable of replication and catalysis.
This RNA-centric stage allowed for primitive evolution, with mutations in nucleotide sequences driving diversity. Transition to DNA genomes provided greater stability, while proteins took over catalytic functions, marking a key evolutionary milestone.
Modern research explores proto-nucleotides, suggesting alternative bases existed early on, influencing life’s chemical foundation. Understanding this helps unravel how complex life emerged from simple molecules.
FAQ 17: What Role Do Nucleotides Play in Viral Genomes?
Viral genomes, whether DNA or RNA, consist of nucleotide sequences that encode essential proteins for replication and host invasion. In RNA viruses like influenza, nucleotides form single-stranded genomes prone to mutations due to error-prone replication, enabling rapid evolution and adaptation.
Nucleotide composition influences viral-host interactions; for example, certain biases in dinucleotide frequencies help viruses evade host immune detection. In DNA viruses like herpes, larger genomes with overlapping nucleotide sequences maximize coding efficiency.
| Viral Genome Type | Nucleotide Features | Implications |
|---|---|---|
| RNA Viruses | High mutation rates, short sequences | Quick adaptation, vaccine challenges |
| DNA Viruses | Stable, larger genomes | Persistent infections, latency |
| Segmented Genomes | Multiple nucleotide segments | Reassortment for new strains |
This table shows how nucleotides drive viral diversity and pathogenesis.
FAQ 18: What Is the Future of Research in Nucleotide Biology?
Future research in nucleotide biology promises breakthroughs in therapeutics, synthetic biology, and understanding cellular mechanisms. Advances in nucleic acid drugs, like improved ASOs and siRNAs, aim to treat rare diseases by precisely targeting genes, with ongoing trials for conditions like Huntington’s.
Integration with AI could predict nucleotide interactions for drug design, accelerating personalized medicine. Expanded genetic alphabets, using synthetic bases, may create novel biomolecules for biotechnology.
Epigenetic studies on nucleotide modifications will reveal roles in aging and disease, paving the way for interventions.
FAQ 19: How Do Nucleotides Influence Immune Responses?
Nucleotides modulate immune function through signaling and structural roles. Extracellular nucleotides like ATP act as danger signals, activating purinergic receptors to trigger inflammation and recruit immune cells during injury.
In innate immunity, nucleotide patterns in microbial DNA/RNA are recognized by toll-like receptors, initiating antiviral responses. Dietary nucleotides enhance adaptive immunity by supporting lymphocyte proliferation.
Dysregulation can lead to autoimmune issues, where self-nucleotides trigger inappropriate responses.
FAQ 20: What Are the Evolutionary Adaptations in Nucleotide Usage Across Species?
Nucleotide usage has evolved to optimize genetic efficiency and adaptation. In prokaryotes, high AT content reduces energy costs for replication, while eukaryotes favor GC-rich regions for stability. Codon bias, influenced by nucleotide availability, affects translation speed.
In viruses, nucleotide mimicry of host patterns evades detection, driving co-evolution. These adaptations underscore nucleotides’ role in life’s diversity.
Acknowledgement
The creation of the article “Three Main Parts of a Nucleotide: Structure and Functions” was made possible through the wealth of scientific knowledge available from various reputable sources. The Examsmeta.com Website extended its gratitude to the following organizations for their comprehensive and accessible resources, which provided critical insights into the structure, functions, and broader implications of nucleotides in biology. Their contributions ensured the article’s accuracy and depth, making complex concepts understandable for a wide audience.
- National Human Genome Research Institute (www.genome.gov): For detailed explanations of nucleotide structure and their role in DNA and RNA.
- Nature Education (www.nature.com): For in-depth resources on nucleotide synthesis and genetic coding.
- Khan Academy (www.khanacademy.org): For clear, educational content on nucleic acids and their functions.
- PubMed Central (www.ncbi.nlm.nih.gov): For peer-reviewed studies on nucleotide metabolism and medical applications.
- Encyclopaedia Britannica (www.britannica.com): For foundational knowledge on molecular biology and nucleotide history.

