Enzymes are fascinating biological molecules that play a crucial role in making life possible. They act as catalysts, speeding up chemical reactions in living organisms without being used up in the process. By lowering the activation energy required for reactions to occur, enzymes enable essential processes like digestion, metabolism, and energy production to happen efficiently. Imagine your body as a bustling factory; enzymes are the workers that keep everything running smoothly, from breaking down food to building new cells. Without them, these reactions would take far too long, and life as we know it wouldn’t exist.
In simple terms, enzymes are mostly proteins made up of long chains of amino acids, folded into specific shapes that allow them to function. They are produced by living organisms, including plants, animals, and microbes, and they catalyze natural processes in the body. Enzymes are often called polypeptides because of their protein nature, and they follow specific mechanisms like the lock-and-key model or the induced fit hypothesis to interact with substrates.
Table of Contents
This article dives deep into everything you need to know about enzymes, from their basic definition to advanced applications in industry and medicine, drawing on established biological principles and practical examples.
What Are Enzymes and Why Are They Important?
Enzymes are nitrogenous organic compounds, primarily proteins, that living organisms produce to facilitate biochemical reactions. They increase the rate of reactions by providing an alternative pathway with lower energy barriers, ensuring that vital processes occur at body temperature. For instance, without enzymes, digesting a meal could take years instead of hours.
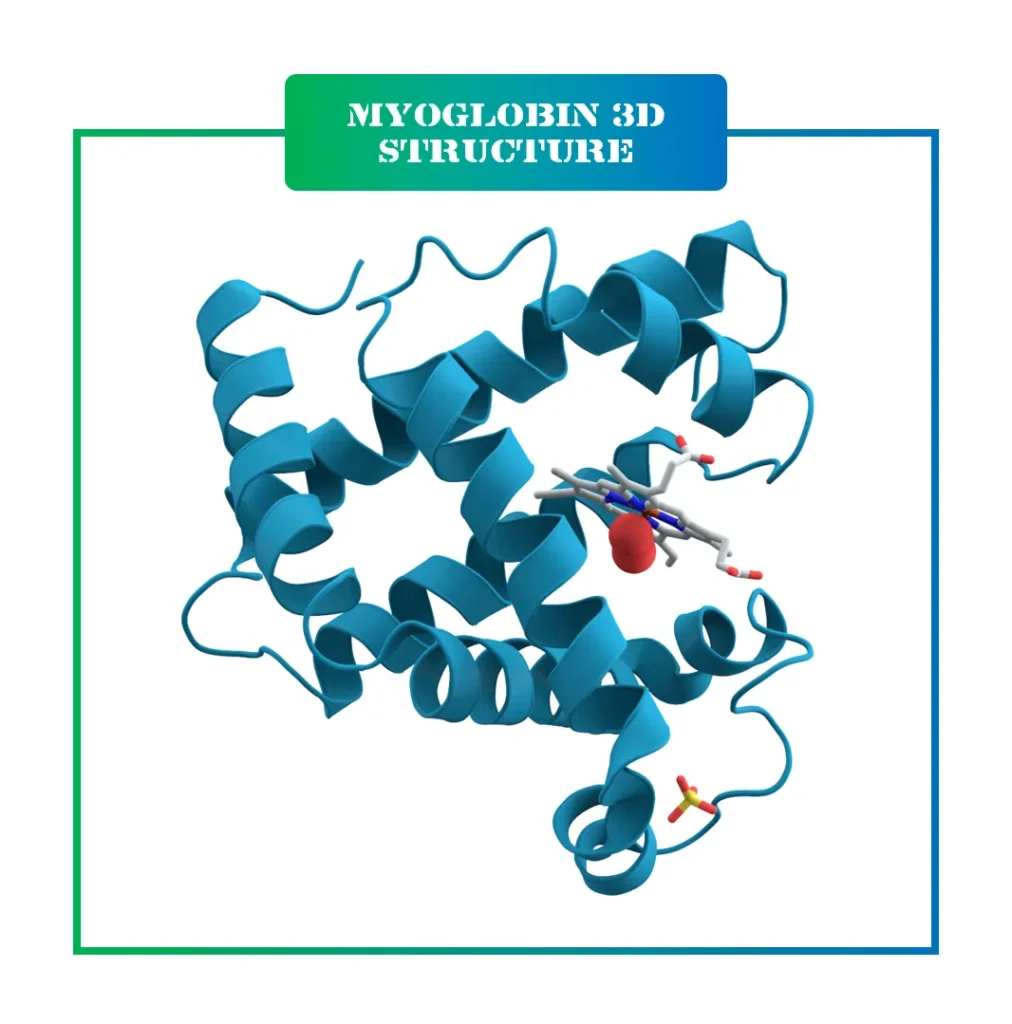
The importance of enzymes extends beyond basic biology. They are central to metabolism, where they help convert food into energy, and to cell signaling, which coordinates body functions. In daily life, enzymes are at work when you eat, breathe, or even think. They also have immense value in biotechnology, where they are harnessed for sustainable manufacturing and medical treatments.
- Key Roles in Living Organisms: Enzymes regulate digestion by breaking down complex molecules into simpler ones, support DNA replication for cell division, and aid in detoxification by neutralizing harmful substances.
- Efficiency Boosters: They can accelerate reactions up to a million times faster than uncatalyzed ones, making them indispensable for life.
- Specificity: Each enzyme typically works on one type of substrate, ensuring precision in biochemical pathways.
Enzymes aren’t just limited to the body; humans have learned to extract and use them in various industries, from food production to pharmaceuticals, highlighting their versatility.
The Intricate Structure of Enzymes
Enzymes are proteins composed of multiple polypeptide chains, which are long sequences of amino acids linked by peptide bonds. These chains fold and coil into complex three-dimensional structures, determining the enzyme’s function. The structure includes linear chains that twist into shapes like helices and sheets, creating a unique form.
A critical part of an enzyme’s structure is the active site, a small region where the substrate binds and the reaction occurs. This site consists of catalytic and binding areas, where specific amino acids interact with the substrate. Only a fraction of the enzyme’s overall structure is involved in catalysis, but the entire folding is essential for stability.
The amino acid sequence, dictated by genes, shapes the enzyme’s catalytic activity. Changes in this sequence can alter function, leading to diseases or improved industrial variants. Some enzymes also incorporate non-protein components, enhancing their structure and activity.
| Structural Component | Description | Example |
|---|---|---|
| Primary Structure | Linear sequence of amino acids | Chain of 100-500 amino acids in amylase |
| Secondary Structure | Local folding into alpha helices or beta sheets | Helices in hemoglobin-like enzymes |
| Tertiary Structure | Overall 3D shape from folding | Globular shape of most enzymes |
| Quaternary Structure | Multiple polypeptide chains assembled | Hemoglobin with four subunits |
| Active Site | Pocket for substrate binding | Cleft in lysozyme for sugar chains |
This table illustrates how enzyme structure builds from basic to complex levels, ensuring precise functionality.
Classification of Enzymes
Enzymes are classified into six categories by the International Union of Biochemistry based on the reactions they catalyze. This system helps organize their diverse functions, from oxidation to bond formation.
Oxidoreductases
These enzymes facilitate oxidation-reduction reactions, transferring electrons between molecules. They often use cofactors like NAD+ or NADP+.
- Function: Catalyze redox processes essential for energy production.
- Example: Pyruvate dehydrogenase converts pyruvate to acetyl-CoA in cellular respiration.
- Equation: AH₂ + B → A + BH₂
Transferases
Transferases move functional groups from one molecule to another.
- Function: Aid in building complex molecules by transferring groups like amino or phosphate.
- Example: Transaminases shift amino groups in amino acid metabolism.
- Equation: A-X + B ↔ B-X + A
Hydrolases
Hydrolases break bonds using water, common in digestion.
- Function: Hydrolyze bonds in proteins, lipids, and carbohydrates.
- Example: Pepsin cleaves peptide bonds in proteins.
- Equation: A-X + H₂O → X-OH + A-H
Lyases
Lyases add or remove groups to form double bonds without hydrolysis or oxidation.
- Function: Involved in metabolic pathways like glycolysis.
- Example: Aldolase splits fructose-1,6-bisphosphate.
- Equation: A-X + B-Y → A = B + X-Y
Isomerases
Isomerases rearrange atoms within a molecule to form isomers.
- Function: Enable quick energy release by shifting groups.
- Example: Phosphoglucomutase converts glucose-1-phosphate to glucose-6-phosphate.
- Equation: A (cis) → A’ (trans)
Ligases
Ligases join two molecules by forming new bonds, often using energy from ATP.
- Function: Essential for DNA repair and synthesis.
- Example: DNA ligase seals DNA fragments.
- Equation: A + B → AB
| Enzyme Class | Reaction Type | Common Cofactor | Biological Role | Industrial Use |
|---|---|---|---|---|
| Oxidoreductases | Oxidation-reduction | NAD+/NADP+ | Energy metabolism | Biofuel production |
| Transferases | Group transfer | None specific | Biosynthesis | Pharmaceutical synthesis |
| Hydrolases | Hydrolysis | Water | Digestion | Detergents for stain removal |
| Lyases | Addition/elimination | None | Catabolism | Food processing |
| Isomerases | Isomerization | None | Metabolic flexibility | Sweetener production |
| Ligases | Bond formation | ATP | DNA replication | Genetic engineering |
This classification table summarizes key aspects, showing how enzymes span biology and applications.
Enzyme Cofactors: The Essential Helpers
Cofactors are non-protein molecules that assist enzymes in catalysis. Without them, many enzymes (apoenzymes) are inactive; together, they form holoenzymes.
There are three main types:
- Prosthetic Groups: Permanently bound, like FAD in oxidoreductases.
- Coenzymes: Loosely bound organic molecules, often vitamin-derived, such as NAD+.
- Metal Ions: Inorganic helpers like Zn²⁺ for coordinate bonds in active sites.
Cofactors enhance specificity and efficiency, playing roles in electron transfer or stabilization. For example, vitamin B deficiencies can impair coenzyme function, leading to health issues.
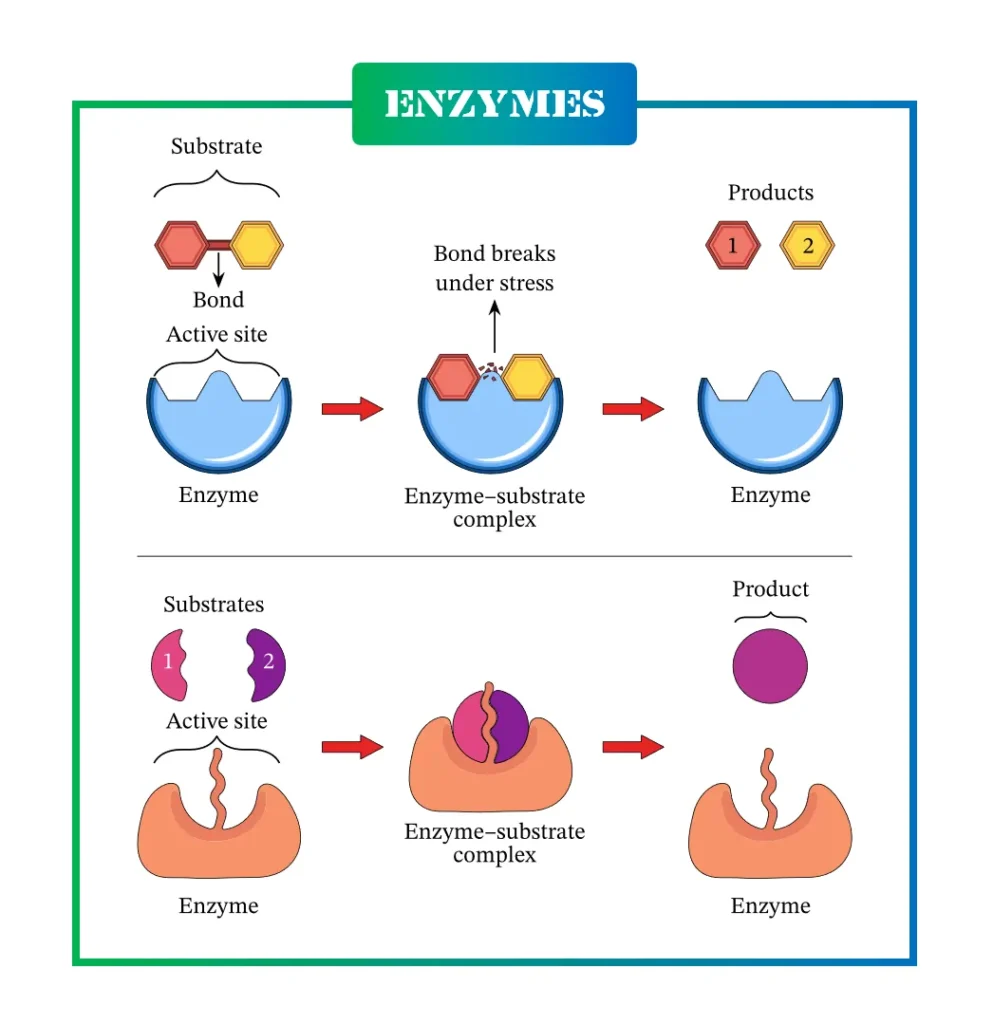
Mechanisms of Enzyme Action: How They Work
Enzyme action involves substrate binding at the active site, forming an enzyme-substrate complex, and product release. This lowers activation energy by stabilizing intermediates.
Two primary models explain this:
Lock and Key Mechanism
Proposed by Emil Fischer in 1894, this model views the active site as a rigid lock and the substrate as a fitting key. The shapes complement each other perfectly, forming a complex for reaction.
- Advantages: Explains specificity.
- Limitations: Doesn’t account for flexibility.
Induced Fit Hypothesis
Daniel Koshland’s 1958 model suggests the active site changes shape upon substrate binding, like a glove molding to a hand. This flexibility improves catalysis.
- Advantages: Better explains conformational changes.
- Example: Hexokinase adapts to glucose.
Both mechanisms highlight how enzymes orient substrates correctly, reducing energy needs.
Enzymes as Biochemical Catalysts
Enzymes are nature’s biochemical catalysts, accelerating reactions without alteration. This property, called catalysis, is harnessed in products like beverages, chocolates, and detergents.
In biotechnology, enzymes enable efficient, eco-friendly processes. For instance, they predigest baby food for easier absorption.
Examples of Enzyme Catalysis in Action
Enzyme catalysis is evident in everyday reactions:
- Cane Sugar Inversion: Invertase converts sucrose to glucose and fructose. Equation: C₁₂H₂₂O₁₁ + H₂O → C₆H₁₂O₆ + C₆H₁₂O₆
- Milk to Curd: Lactase from lactobacilli ferments lactose.
- Glucose to Ethanol: Zymase in yeast produces alcohol and CO₂. Equation: C₆H₁₂O₆ → 2C₂H₅OH + 2CO₂
- Starch to Maltose: Diastase breaks down starch in brewing.
These examples show enzymes’ role in food and fermentation.
In medicine, enzymes like streptokinase dissolve blood clots.
Factors Affecting Enzyme Catalysis
Several factors influence enzyme activity:
- Substrate Concentration: Reaction rate increases until saturation.
- Enzyme Concentration: Directly proportional to rate if substrate is abundant.
- Temperature: Optimal around 37°C for human enzymes; higher causes denaturation.
- pH: Most work best at neutral pH; extremes ionize side chains, reducing activity.
- Inhibitors: Slow or stop reactions.
| Factor | Optimal Range | Effect of Deviation | Example |
|---|---|---|---|
| Substrate Concentration | Varies by enzyme | Saturation limits rate | High glucose speeds amylase |
| Enzyme Concentration | Proportional to substrate | Rate increases linearly | More pepsin digests faster |
| Temperature | 30-40°C for most | Denaturation above 50°C | Body fever slows enzymes |
| pH | 6-8 for many | Inactivation in acids/bases | Pepsin at pH 2 in stomach |
| Inhibitors | None | Reduces activity | Cyanide poisons cytochrome oxidase |
This table details how conditions optimize or hinder enzymes.
Inhibition of Enzymes: Control and Regulation
Inhibition regulates enzyme activity to maintain balance. Types include:
- Competitive Inhibition: Inhibitor competes for active site.
- Non-Competitive Inhibition: Binds elsewhere, altering shape.
- Uncompetitive Inhibition: Binds to enzyme-substrate complex.
- Irreversible Inhibition: Permanently inactivates, like poisons.
Feedback inhibition, where products inhibit earlier enzymes, prevents overproduction.
Drug Action on Enzymes
Many drugs target enzymes as inhibitors or activators. Competitive inhibitors block active sites, like statins reducing cholesterol synthesis.
In cancer treatment, kinase inhibitors halt cell growth. Enzyme replacement therapy treats deficiencies, such as in Gaucher’s disease.
Specific Examples of Enzymes
Here are common enzymes and their roles:
- Lipases: Digest fats in the gut.
- Amylase: Breaks starches into sugars in saliva.
- Maltase: Converts maltose to glucose.
- Trypsin: Cleaves proteins in the intestine.
- Lactase: Hydrolyzes lactose in milk.
- Helicase: Unwinds DNA.
- DNA Polymerase: Synthesizes DNA.
In industry, proteases tenderize meat, and cellulases soften fabrics.
The Chemical Nature of Enzymes
Enzymes are primarily proteins, chains of amino acids folded into functional shapes. The active site relies on this 3D structure for substrate binding.
Some are RNA molecules (ribozymes), catalyzing reactions like RNA splicing. Proteins dominate due to diversity from 20 amino acids.
Applications of Enzymes in Industry and Medicine
Enzymes revolutionize industries by enabling green processes.
In food: Amylase improves baking, lactase makes lactose-free milk.
In detergents: Proteases remove protein stains in cold water, saving energy.
In medicine: Proteases heal burns, fibrinolytics bust clots. Diagnostics use enzymes for blood sugar tests.
Biofuels: Cellulases break plant material into ethanol.
| Industry | Enzyme Used | Application | Benefit |
|---|---|---|---|
| Food Processing | Amylase, Protease | Bread baking, cheese making | Improved texture, flavor |
| Detergents | Lipase, Cellulase | Stain removal, fabric softening | Eco-friendly cleaning |
| Pharmaceuticals | Kinase Inhibitors | Cancer treatment | Targeted therapy |
| Biofuels | Cellulase | Biomass conversion | Sustainable energy |
| Textiles | Pectinase | Cotton scouring | Reduced water use |
This table showcases practical uses, emphasizing sustainability.
Enzymes in Daily Life
Everyday, enzymes impact us unknowingly. In the kitchen, yeast enzymes ferment dough for bread. In health, digestive enzymes supplements aid those with deficiencies.
In agriculture, enzymes enhance animal feed digestion, improving nutrition. Even in recycling, they break down paper for reuse.
Understanding enzymes not only reveals biological wonders but also inspires innovations for a better future. Whether in our bodies or factories, these catalysts drive progress efficiently and naturally.
Read These Articles in Detail
- The Cell: The Fundamental Building Block of Life
- Prokaryotic Cells vs. Eukaryotic Cells: A Detailed Exploration
- Prokaryotic Cells: Definition, Anatomy, Functions, and Their Importance
- Eukaryotic Cells Explained: Definition, Structure, Functions, and Importance
- The Cell Membrane: Structure, Functions, and Evolutionary Significance
- The Cell Wall: Structure, Composition, Functions, and Evolutionary Significance
- Endomembrane System: A Detailed Exploration of the Cellular Machinery of Life
- Mitochondria: A Comprehensive Guide to Their Structure and Functions
- The Cell Envelope: The Protective Shield of Prokaryotic Cells
- Ribosomes: Definition, Structure, and Functions
- Endoplasmic Reticulum: Structure, Types, and Functions Explained
- The Golgi Apparatus: Structure, Functions, and Cellular Importance
- Cytoplasm and Nucleus: Structure, Functions, Differences, and Examples
- What Is Cell Signaling? Structure, Types, and Functions Explained
- Three Main Parts of a Nucleotide: Structure and Functions
- Enzymes: Definition, Structure, Classification, and Functions
- DNA: Structure, Function, Discovery, and Modern Applications
- DNA Replication: Steps, Process, and Genetic Importance
- Properties of DNA: Structure, Physical, and Chemical Characteristics
- DNA Packaging: How DNA Fits into the Nucleus of a Cell
- R-Factors and Plasmids: How Bacteria Develop Antibiotic Resistance
- Chromosomes: Structure, Types, Functions, and Role in Genetics
- Genetic Material: DNA, RNA, and Their Role in Heredity and Evolution
- Deoxyribose and Ribose: Structure and Function in DNA and RNA
- Differences Between Genes and DNA: Definitions, Structures, and Functions
- RNA: Definition, Structure, Types, Functions, and Its Role in Life
Frequently Asked Questions
FAQ 1: What Are Enzymes and Why Are They Important?
Enzymes are biological catalysts that speed up chemical reactions in living organisms without being consumed in the process. These special molecules, usually proteins, lower the activation energy needed for reactions, allowing processes like digestion, metabolism, and DNA replication to occur quickly and efficiently. Without enzymes, these reactions would be too slow to sustain life, as they would require much higher temperatures or energy levels that living organisms cannot provide.
The importance of enzymes extends beyond the body. They play a critical role in industries such as food production, where enzymes like amylase help bake better bread, and in medicine, where enzymes like streptokinase dissolve blood clots. Their ability to catalyze reactions with high specificity makes them essential for both natural processes and technological advancements. For example, enzymes in detergents break down stains, making cleaning more eco-friendly. By enabling reactions at mild conditions, enzymes contribute to sustainable practices, reducing energy use in industrial processes.
FAQ 2: How Is the Structure of Enzymes Formed?
Enzymes are primarily proteins made up of long chains of amino acids linked by peptide bonds. These chains fold into complex three-dimensional structures, which are critical for their function. The structure is organized into several levels: the primary structure (the linear sequence of amino acids), the secondary structure (local folding into shapes like alpha helices or beta sheets), the tertiary structure (the overall 3D shape), and in some cases, the quaternary structure (multiple polypeptide chains working together).
A key feature of an enzyme’s structure is its active site, a small pocket where the substrate binds and the reaction occurs. The active site’s shape and chemical properties are determined by the amino acid sequence, ensuring specificity. Some enzymes also include cofactors, non-protein molecules like metal ions or coenzymes, which enhance their activity. This intricate structure allows enzymes to perform precise tasks, such as breaking down sugars or synthesizing DNA, with remarkable efficiency.
FAQ 3: How Are Enzymes Classified?
Enzymes are classified into six main categories by the International Union of Biochemistry, based on the type of reaction they catalyze. These include oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. Each group has a unique role in biochemical processes, making classification essential for understanding their functions.
For example, oxidoreductases handle oxidation-reduction reactions, like pyruvate dehydrogenase in energy production, while hydrolases break bonds using water, as seen with pepsin in digestion. Transferases move functional groups, lyases form double bonds, isomerases rearrange molecules, and ligases join molecules using energy from ATP. This system helps scientists and industries identify enzymes for specific tasks, from brewing beer with diastase to repairing DNA with DNA ligase.
FAQ 4: What Are Enzyme Cofactors and Their Role?
Cofactors are non-protein molecules that assist enzymes in catalyzing reactions. Without them, some enzymes, called apoenzymes, cannot function. When combined with a cofactor, they form a holoenzyme, which is fully active. Cofactors can be prosthetic groups (permanently bound, like FAD), coenzymes (organic molecules like NAD+ that bind temporarily), or metal ions (like Zn²⁺) that stabilize reactions.
Cofactors enhance enzyme efficiency by facilitating electron transfers or stabilizing substrates. For instance, in cellular respiration, NAD+ helps oxidoreductases transfer electrons. Deficiencies in cofactors, such as those derived from vitamins, can impair enzyme function, leading to health issues. In industry, understanding cofactors allows for optimized enzyme use, such as in biofuel production, where metal ions improve enzyme stability and activity.
FAQ 5: How Do Enzymes Work Through the Lock and Key Mechanism?
The lock and key mechanism, proposed by Emil Fischer in 1894, explains how enzymes interact with substrates. In this model, the enzyme’s active site is like a lock, perfectly shaped to fit the substrate, which acts as the key. This precise fit ensures that only the correct substrate binds, leading to a specific reaction. For example, maltase breaks down maltose into glucose because their shapes match perfectly.
While this model highlights enzyme specificity, it assumes a rigid active site, which isn’t always accurate. The lock and key mechanism is widely used to explain simple enzyme-substrate interactions, such as in digestion, where trypsin targets specific protein bonds. Its simplicity makes it a great starting point for understanding enzyme function, though newer models like the induced fit hypothesis account for flexibility in the active site.
FAQ 6: What Is the Induced Fit Hypothesis?
The induced fit hypothesis, proposed by Daniel Koshland in 1958, suggests that the enzyme’s active site is not rigid but flexible, molding to the substrate like a glove to a hand. When the substrate binds, the active site adjusts its shape to fit more tightly, enhancing catalysis. This model explains why enzymes can sometimes bind slightly mismatched substrates and still function effectively.
For example, hexokinase changes shape when binding glucose, ensuring efficient phosphorylation in energy metabolism. The induced fit model accounts for the dynamic nature of enzymes, making it a more comprehensive explanation than the lock and key mechanism. It’s particularly relevant in complex reactions, such as those in DNA replication, where enzymes like DNA polymerase adapt to varying substrates to maintain accuracy.
FAQ 7: What Factors Affect Enzyme Activity?
Enzyme activity depends on several factors that influence their efficiency. Substrate concentration increases reaction rates until the enzyme is saturated, while enzyme concentration boosts rates if substrates are abundant. Temperature is critical, with most human enzymes working best around 37°C; too high, and the enzyme denatures, losing its shape. pH also matters, as enzymes like pepsin thrive in acidic environments (pH 2), while others prefer neutral conditions.
Inhibitors can slow or stop enzyme activity. Competitive inhibitors block the active site, while non-competitive inhibitors bind elsewhere, altering the enzyme’s shape. For instance, cyanide poisons cytochrome oxidase, halting energy production. Understanding these factors helps optimize enzymes in medicine, where drugs target specific enzymes, and in industry, where conditions are controlled for maximum output.
FAQ 8: How Are Enzymes Used in Industry?
Enzymes are vital in industries due to their efficiency and eco-friendly nature. In food processing, amylase improves bread texture, and lactase creates lactose-free milk for those with lactose intolerance. Proteases in detergents break down protein stains, allowing effective cleaning at lower temperatures, which saves energy. In biofuels, cellulases convert plant material into ethanol, supporting sustainable energy production.
In textiles, pectinases soften cotton fibers, reducing water and chemical use. The pharmaceutical industry uses enzymes like kinase inhibitors for cancer treatments, targeting specific pathways. Enzymes enable precise, green processes, making them invaluable for reducing environmental impact while meeting consumer needs, from better-tasting cheese to cleaner laundry.
FAQ 9: How Do Enzymes Contribute to Medicine?
Enzymes play a transformative role in medicine, both in treatments and diagnostics. Enzyme replacement therapy treats conditions like Gaucher’s disease by supplying missing enzymes. Streptokinase and similar enzymes dissolve blood clots in heart attack patients, saving lives by restoring blood flow. In diagnostics, enzymes like glucose oxidase are used in blood sugar tests for diabetes management, providing quick and accurate results.
Enzyme inhibitors are also key in drug design. Statins, for example, inhibit enzymes involved in cholesterol synthesis, lowering heart disease risk. By targeting specific enzymes, drugs can regulate biochemical pathways with precision, minimizing side effects. Ongoing research continues to uncover new enzyme-based therapies, highlighting their potential to address complex health challenges.
FAQ 10: What Are Some Common Examples of Enzymes in the Body?
The human body relies on numerous enzymes for survival. Lipases in the intestines break down fats into absorbable molecules, while amylase in saliva converts starches into sugars for energy. Maltase further processes maltose into glucose, found in foods like pasta. Trypsin, active in the small intestine, digests proteins into amino acids, essential for tissue repair and growth.
Lactase helps digest lactose in milk, preventing discomfort in lactose-tolerant individuals. In DNA-related processes, helicase unwinds the DNA double helix, and DNA polymerase builds new DNA strands during replication. These enzymes ensure that vital functions, from digestion to genetic replication, occur seamlessly, supporting overall health and well-being.
FAQ 11: How Have Enzymes Evolved Over Time?
Enzymes have been evolving for billions of years, shaping the chemical reactions that sustain life on Earth. From the earliest forms of life, these biological catalysts have adapted through natural selection to perform increasingly complex tasks. Early enzymes likely emerged in the primordial soup, where simple molecules began catalyzing reactions essential for primitive metabolism. Over time, as organisms diversified, enzymes evolved to handle new substrates and environments, leading to the vast array of functions we see today in processes like photosynthesis and cellular respiration.
The evolution of enzymes often occurs through mechanisms like gene duplication, where a copy of an enzyme’s gene mutates to acquire new functions while the original retains its role. This allows for functional diversity within enzyme superfamilies, where related enzymes share a common ancestor but catalyze different reactions. For instance, oxidoreductases have evolved to transfer electrons in various metabolic pathways, adapting to oxygen-rich atmospheres that emerged later in Earth’s history. Directed evolution, a lab technique mimicking natural processes, accelerates this by introducing mutations and selecting for improved traits, such as stability or novel activities.
Human understanding of enzyme evolution has advanced through bioinformatics, analyzing vast sequence data to trace evolutionary paths. Studies show that enzymes can evolve new functions easily, but optimizing them for efficiency is complex, requiring fine-tuned changes in active sites. Convergent evolution is another fascinating aspect, where unrelated enzymes develop similar catalytic mechanisms for the same reaction, highlighting nature’s ingenuity. This evolutionary flexibility underscores why enzymes are so versatile, from breaking down toxins in bacteria to aiding digestion in humans.
Looking ahead, insights from enzyme evolution inform biotechnology, where scientists engineer enzymes for industrial uses, drawing on billions of years of natural refinement to create sustainable solutions.
FAQ 12: What Happens When Enzymes Are Deficient in the Body?
Enzyme deficiencies can lead to a range of health issues, often stemming from genetic mutations that impair enzyme production or function. These conditions disrupt normal metabolic processes, causing buildup of toxic substances or shortages of essential molecules. Understanding these deficiencies helps in diagnosing and managing related diseases.
| Deficiency Type | Associated Diseases | Causes | Symptoms | Treatments |
|---|---|---|---|---|
| Lysosomal Storage Disorders | Gaucher Disease, Fabry Disease | Genetic mutations leading to missing or faulty enzymes that break down fats or sugars | Organ enlargement, pain, fatigue, neurological issues | Enzyme replacement therapy (ERT), substrate reduction therapy |
| Metabolic Disorders | Phenylketonuria (PKU), Ornithine Transcarbamylase Deficiency | Inherited gene defects affecting amino acid or urea cycle enzymes | Intellectual disability, seizures, high ammonia levels | Dietary restrictions, medications to remove toxins, liver transplant in severe cases |
| Digestive Enzyme Insufficiencies | Pancreatic Insufficiency, Lactose Intolerance | Chronic pancreatitis, cystic fibrosis, or genetic lack of lactase | Malnutrition, diarrhea, bloating | Oral enzyme supplements, dietary changes |
| Blood-Related Deficiencies | Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency | X-linked genetic mutation | Anemia triggered by infections or certain foods | Avoidance of triggers, supportive care like transfusions |
| Intestinal Transporter Disorders | Congenital Sucrase-Isomaltase Deficiency | Mutations in genes for carbohydrate-digesting enzymes | Chronic diarrhea, failure to thrive | Enzyme supplements, low-sugar diets |
This table highlights common enzyme deficiencies, emphasizing the need for early detection through genetic screening to improve outcomes.
FAQ 13: How Are Enzymes Engineered for Better Performance?
Enzyme engineering involves modifying enzymes to enhance their properties, making them more suitable for industrial, medical, or environmental applications. Techniques like directed evolution and rational design allow scientists to tweak enzyme structures for improved stability, activity, or specificity.
- Directed Evolution: This method mimics natural selection by introducing random mutations and screening for desired traits. It’s particularly useful for creating enzymes with novel functions, such as those used in biofuel production.
- Rational Design: Using computational models, researchers predict and alter specific amino acids in the active site to optimize performance, like increasing thermostability for high-temperature processes.
- Site-Directed Mutagenesis: Targets precise changes in the enzyme’s DNA sequence, enabling fine-tuned improvements in substrate binding or catalytic efficiency.
- Semi-Rational Design: Combines random mutagenesis with targeted alterations, accelerating the discovery of superior variants.
- Machine Learning Integration: Recent advances use AI to predict enzyme behaviors, speeding up design for applications in drug synthesis or waste degradation.
These approaches have revolutionized fields like pharmaceuticals, where engineered enzymes produce complex molecules more efficiently.
FAQ 14: What Role Do Enzymes Play in Environmental Sustainability?
Enzymes are pivotal in promoting environmental sustainability by enabling eco-friendly processes that reduce waste and energy consumption. In bioremediation, enzymes break down pollutants like pesticides and plastics, restoring contaminated soils and waters. For example, engineered enzymes target toxic chemicals, converting them into harmless substances, which is crucial for cleaning up industrial sites.
In the realm of biofuels, enzymes like cellulases convert plant biomass into ethanol, offering a renewable alternative to fossil fuels and lowering carbon emissions. This process is more efficient and less polluting than traditional methods, aligning with global efforts to combat climate change. Enzymes also facilitate sustainable manufacturing in industries such as textiles and paper, where they replace harsh chemicals, minimizing environmental impact.
Furthermore, in waste management, enzymes accelerate the decomposition of organic matter in composting and wastewater treatment, reducing methane emissions from landfills. Their biodegradability ensures no residual harm to ecosystems. As research progresses, enzymes are being optimized for carbon capture, where they help sequester CO2, contributing to climate mitigation strategies. Overall, harnessing enzymes fosters a greener future by supporting circular economies and reducing reliance on non-renewable resources.
FAQ 15: Can Enzymes Be Used in Household Cleaning?
Enzymes are increasingly incorporated into household cleaning products for their ability to target specific stains effectively while being gentle on surfaces and the environment. They break down organic matter at a molecular level, making cleaning more efficient.
| Enzyme Type | Function in Cleaning | Common Products | Benefits | Examples of Stains Targeted |
|---|---|---|---|---|
| Proteases | Break down protein-based stains | Laundry detergents, dish soaps | Removes tough residues without harsh chemicals | Blood, grass, food proteins |
| Amylases | Degrade starches | Dishwasher detergents, carpet cleaners | Prevents starch buildup | Pasta, rice, potato stains |
| Lipases | Target fats and oils | Multi-surface cleaners, grease removers | Effective on oily messes | Butter, oil, lipstick |
| Cellulases | Soften fabrics by breaking cellulose | Laundry boosters | Maintains fabric integrity | Dirt on cotton clothes |
| Mannanases | Remove gum-like substances | Gum removers, detergents | Tackles sticky residues | Guar gum in foods or cosmetics |
This table demonstrates how enzymes enhance everyday cleaning, promoting sustainability by allowing lower wash temperatures and reducing chemical use.
FAQ 16: How Do Enzymes Interact with Other Biomolecules?
Enzymes interact with various biomolecules to catalyze reactions efficiently, forming temporary complexes that facilitate biochemical processes. These interactions are highly specific, ensuring precise control in cellular environments.
- Substrate Binding: Enzymes bind substrates at the active site through weak interactions like hydrogen bonds and van der Waals forces, lowering activation energy.
- Cofactor Involvement: Many enzymes require cofactors, such as metal ions or coenzymes, to stabilize intermediates or transfer groups during reactions.
- Protein-Protein Interactions: Enzymes often form complexes with other proteins, enhancing regulation in pathways like signal transduction.
- Lipid Membrane Associations: Some enzymes anchor to membranes, where lipid interactions modulate activity, as in regulatory mechanisms.
- Nucleic Acid Interactions: Enzymes like DNA polymerase interact with DNA, ensuring accurate replication through base-pairing and proofreading.
These dynamic interactions highlight enzymes’ role in maintaining life’s complexity.
FAQ 17: What Are Ribozymes and How Do They Differ from Protein Enzymes?
Ribozymes are RNA molecules that act as catalysts, similar to protein enzymes but composed of nucleic acids rather than amino acids. Discovered in the 1980s, they challenge the traditional view that all enzymes are proteins, supporting theories like the RNA world hypothesis, where RNA served dual roles in early life.
Unlike protein enzymes, which fold into diverse 3D structures from 20 amino acids, ribozymes rely on RNA’s four nucleotides, limiting their structural complexity but allowing self-splicing in processes like mRNA maturation. Protein enzymes generally exhibit higher catalytic efficiency and versatility due to their broader chemical repertoire.
Ribozymes excel in specific reactions, such as cleaving phosphodiester bonds in RNA, while protein enzymes handle a wider array, including oxidation and hydrolysis. However, both lower activation energy by stabilizing transition states. This difference underscores evolutionary insights, with ribozymes possibly predating proteins.
FAQ 18: How Are Enzymes Measured and Assayed?
Enzyme assays are essential for quantifying activity, aiding in research and diagnostics. Various methods detect changes in substrates or products, providing insights into kinetics and inhibition.
| Assay Method | Description | Applications | Advantages | Limitations |
|---|---|---|---|---|
| Spectrophotometric | Measures absorbance changes from colored products or substrates | Kinetic studies of dehydrogenases | Fast, quantitative | Requires chromogenic compounds |
| Fluorometric | Detects fluorescence intensity shifts | High-sensitivity assays for low-concentration enzymes | Highly sensitive | Potential interference from quenching |
| Chromatographic | Separates and quantifies reaction components | Complex mixture analysis | Precise separation | Time-consuming |
| Manometric | Tracks gas production or consumption | Respiration enzymes | Direct measurement | Limited to gas-involved reactions |
| Electrode-Based | Uses sensors for ion or pH changes | pH-dependent enzymes | Real-time monitoring | Electrode maintenance required |
This table covers key techniques, emphasizing their role in understanding enzyme behavior.
FAQ 19: What Is Enzyme Kinetics and Why Is It Important?
Enzyme kinetics studies how reaction rates change with conditions like substrate concentration, providing a framework for understanding catalysis. The Michaelis-Menten equation is central, describing saturation kinetics where rate increases with substrate until maximum velocity is reached.
- Key Parameters: Km (Michaelis constant) indicates substrate affinity; lower Km means higher affinity. Vmax represents maximum rate at saturation.
- Importance in Medicine: Kinetics guide drug design, as inhibitors alter Km or Vmax, treating conditions like high cholesterol.
- Industrial Applications: Optimizes enzyme use in processes like fermentation, ensuring efficiency.
- Regulatory Insights: Reveals inhibition types, aiding pathway control studies.
- Experimental Tools: Lineweaver-Burk plots linearize data for parameter estimation.
Kinetics is vital for biotechnology and pharmacology advancements.
FAQ 20: What Are the Latest Advances in Enzyme Research?
Enzyme research in 2025 has seen remarkable progress, driven by AI and biotechnology. Scientists are designing de novo enzymes from scratch, creating catalysts for reactions not found in nature, such as efficient carbon fixation for climate solutions. These AI-guided designs tailor active sites for specificity, revolutionizing drug manufacturing and chemical synthesis.
Another breakthrough involves photobiocatalysis, where enzymes harness light for new reactions, expanding applications in sustainable chemistry. In medicine, enzyme therapies target metabolic disorders more precisely, with improved stability for longer efficacy.
Environmental applications have advanced with enzymes for plastic degradation and biofuel production, reducing reliance on fossil fuels. Projects like FUTURENZYME develop enzymes for greener consumer products, enhancing detergent efficiency. Overall, these innovations promise transformative impacts on health, industry, and sustainability.
Acknowledgement
The Examsmeta.com Website sincerely expresses its gratitude to the following reputable sources for providing valuable insights and data that enriched the article “Enzymes: Definition, Structure, Classification, and Functions.” Their comprehensive resources on enzyme biology, applications, and advancements were instrumental in crafting a detailed and informative piece. Examsmeta sincerely appreciates their contributions to scientific knowledge, which helped us present a well-rounded exploration of enzymes.
- National Center for Biotechnology Information (NCBI): For in-depth biochemical data and research on enzyme structure and function.
- ScienceDirect (ScienceDirect): For peer-reviewed articles on enzyme kinetics, engineering, and industrial applications.
- Nature (Nature): For cutting-edge research on enzyme evolution and recent advancements in biocatalysis.
- PubMed (PubMed): For medical and clinical insights into enzyme deficiencies and therapeutic uses.
- Royal Society of Chemistry (RSC): For detailed information on enzyme applications in sustainable chemistry and environmental solutions.

