Life as we know it hinges on tiny molecules that carry the instructions for everything from eye color to how our cells function. At the heart of this genetic machinery are two simple sugars: deoxyribose and ribose. These pentose sugars might seem similar at first glance, but their subtle differences play a massive role in distinguishing DNA from RNA, the two main types of nucleic acids.
In this comprehensive guide, we’ll dive deep into what makes deoxyribose and ribose unique, exploring their structures, histories, biological importance, and much more. Whether you’re a student brushing up on biology or just curious about the building blocks of life, this article breaks it all down in straightforward terms.
Table of Contents
We’ll start with the basics and build up to more advanced insights, drawing from established scientific knowledge to paint a full picture. By the end, you’ll have a clear understanding of why these sugars are so vital and how their differences impact everything from genetic stability to cellular processes.
Introduction to Pentose Sugars in Nucleic Acids
Pentose sugars are five-carbon carbohydrates that form the backbone of nucleic acids, which are essential for storing and transmitting genetic information. Deoxyribose and ribose are both aldopentoses, meaning they have an aldehyde group and five carbon atoms. They undergo phosphorylation to become part of nucleotides, the basic units of DNA and RNA.
In simple terms, nucleotides are like Lego bricks: each has a sugar, a phosphate group, and a nitrogenous base. These bricks link up to form the long chains of DNA and RNA. The sugar in DNA is deoxyribose, while RNA uses ribose. This distinction isn’t just a minor detail; it affects how stable the molecules are and what roles they play in the body. For instance, DNA’s deoxyribose makes it more resistant to breakdown, perfect for long-term storage of genetic info, whereas RNA’s ribose allows for more flexibility in short-term tasks like protein synthesis.
Beyond genetics, these sugars have broader implications. They help enzymes in our bodies tell DNA and RNA apart, ensuring that cellular processes run smoothly. Imagine if your body’s blueprint got mixed up; that’s why these differences matter so much.
What is Ribose?
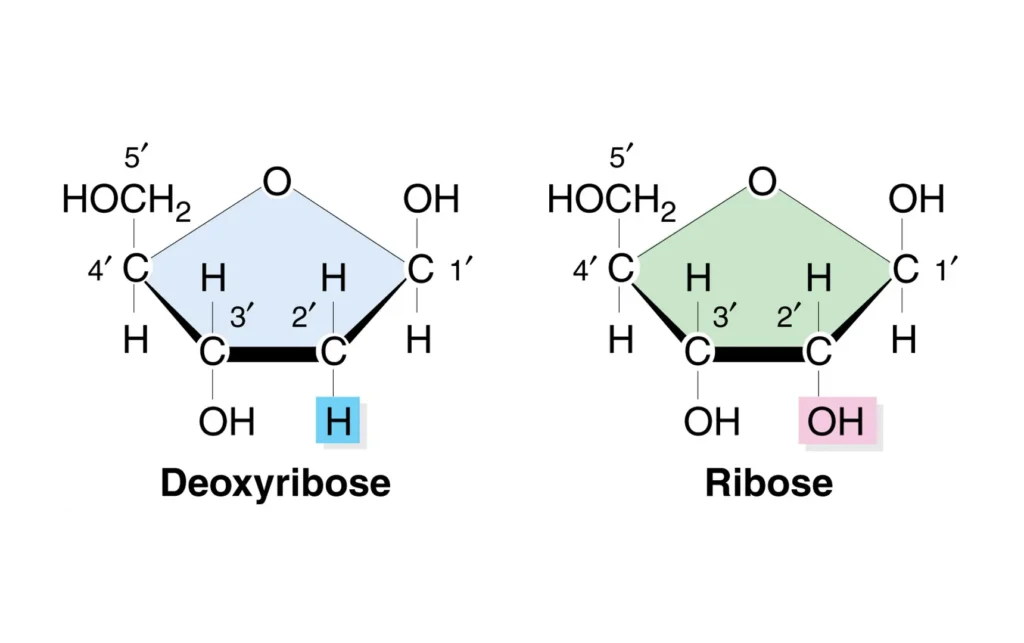
Ribose is a simple sugar, or monosaccharide, that’s crucial for life. It’s an aldopentose with the chemical formula C5H10O5. In its open-chain form, it has an aldehyde group at one end, but in cells, it usually forms a five-membered ring structure called a furanose ring. This ring includes four carbon atoms and one oxygen, with various hydroxyl groups attached.
One key feature of ribose is the hydroxyl (OH) group at the 2′ carbon position. This extra oxygen makes ribose more reactive than its counterpart. Ribose can exist in two anomeric forms: alpha and beta, depending on the orientation of the OH group at the anomeric carbon (carbon 1). The beta form is more common in biological systems.
In the body, ribose combines with a nitrogenous base to form a ribonucleoside, and when a phosphate group is added, it becomes a ribonucleotide. These are the building blocks of RNA, which is involved in decoding genetic information from DNA to make proteins.
Ribose isn’t just limited to RNA; it’s also part of important molecules like ATP (adenosine triphosphate), the energy currency of cells. Without ribose, our cells couldn’t efficiently transfer energy or synthesize proteins.
Properties of Ribose
Let’s break down some key properties:
- Molar Mass: 150.13 g/mol, slightly higher due to the extra oxygen atom.
- IUPAC Name: (2S,3R,4S,5R)-5-(hydroxymethyl)oxolane-2,3,4-triol.
- Solubility: Highly soluble in water, which is essential for its role in aqueous cellular environments.
- Reactivity: The 2′ OH group makes it prone to hydrolysis, meaning RNA breaks down more easily than DNA.
Ribose’s structure allows it to fold into complex shapes, which is why RNA can form loops and hairpins for functions like catalysis in ribozymes.
What is Deoxyribose?
Deoxyribose, on the other hand, is the sugar found in DNA. Its chemical formula is C5H10O4, missing one oxygen compared to ribose. Like ribose, it’s an aldopentose that forms a furanose ring, but the key difference is at the 2′ carbon: instead of an OH group, there’s just a hydrogen atom. This “deoxy” modification (meaning without oxygen) is what gives it its name.
Deoxyribose was first synthesized in 1935 but wasn’t isolated from DNA until 1954. In DNA, it alternates with phosphate groups to form the sturdy sugar-phosphate backbone, to which nitrogenous bases like adenine, thymine, guanine, and cytosine attach.
This sugar’s structure helps DNA maintain its double-helix form, discovered by Watson and Crick in 1953. The lack of the 2′ OH group makes DNA less reactive and more stable, ideal for storing genetic information over long periods.
Deoxyribose also plays a role in distinguishing DNA from RNA at the enzymatic level, preventing mix-ups in cellular processes.
Properties of Deoxyribose
Here are some standout properties:
- Molar Mass: 134.13 g/mol, lighter without the extra oxygen.
- IUPAC Name: 2-deoxy-D-ribose.
- Stability: More resistant to chemical breakdown, thanks to the missing OH group.
- Also Known As: 2-deoxy-D-erythro-pentose.
In lab settings, deoxyribose can be used in tests like the diphenylamine reaction to detect DNA.
Structural Differences Between Deoxyribose and Ribose
The structures of deoxyribose and ribose are incredibly similar, both being five-carbon rings with hydroxyl groups. But that one tiny change at the 2′ position a hydrogen in deoxyribose versus an OH in ribose changes everything.
In ribose, the 2′ OH group adds an extra layer of reactivity. This makes RNA single-stranded and capable of folding into functional shapes, like transfer RNA (tRNA) that carries amino acids during protein synthesis. Deoxyribose’s lack of this group allows DNA to form a stable double helix, where two strands pair up via hydrogen bonds between bases.
Visually, if you imagine the ring, ribose has OH groups at 1′, 2′, 3′, and 5′ (with 5′ being the hydroxymethyl), while deoxyribose skips the 2′. This structural tweak evolved to suit their roles: stability for DNA, versatility for RNA.
Detailed Comparison Table of Structures
| Aspect | Deoxyribose | Ribose |
|---|---|---|
| Ring Type | Furanose (five-membered) | Furanose (five-membered) |
| Carbon 1 Attachment | Aldehyde in open form, anomeric in ring | Same as deoxyribose |
| Carbon 2 Group | Hydrogen (H) | Hydroxyl (OH) |
| Carbon 3 Group | Hydroxyl (OH) | Hydroxyl (OH) |
| Carbon 4 Group | CH2OH (hydroxymethyl) | CH2OH (hydroxymethyl) |
| Anomeric Forms | Alpha and beta | Alpha and beta |
| Open-Chain Form | Aldehyde with four OH groups | Aldehyde with five OH groups |
This table highlights how a single atom swap leads to profound functional differences.
Chemical Formulas and Molar Masses
Chemically, ribose is C5H10O5, while deoxyribose is C5H10O4. That extra oxygen in ribose accounts for its higher molar mass of 150.13 g/mol compared to deoxyribose’s 134.13 g/mol.
In terms of naming, ribose’s full IUPAC name reflects its triol structure, emphasizing the multiple OH groups. Deoxyribose’s name points directly to its modification from ribose.
These formulas are key in lab synthesis and analysis. For example, in biochemistry experiments, the difference in oxygen allows for specific staining techniques to identify DNA versus RNA in cells.
Historical Discovery and Milestones
The story of these sugars is tied to the broader history of nucleic acids. Ribose was discovered in 1891 by Emil Fischer and Oskar Piloty, who were pioneering carbohydrate chemistry. Fischer’s work on sugars earned him a Nobel Prize in 1902.
Deoxyribose came later, identified by Phoebus Levene in 1929. Levene was studying nucleic acids and realized DNA had a different sugar. It was synthesized in 1935 but isolated from actual DNA in 1954.
The bigger picture includes Friedrich Miescher’s 1869 discovery of “nuclein,” later renamed nucleic acid. Albrecht Kossel identified components like deoxyribose, and experiments by Griffith (1928) and Avery (1944) proved DNA as genetic material. Watson and Crick’s 1953 double-helix model sealed the deal, using data from Rosalind Franklin.
These discoveries weren’t just academic; they paved the way for modern genetics, from CRISPR editing to personalized medicine.
Timeline of Key Discoveries
| Year | Event | Key Figure(s) |
|---|---|---|
| 1869 | Discovery of nuclein (precursor to DNA) | Friedrich Miescher |
| 1891 | Discovery of ribose | Emil Fischer and Oskar Piloty |
| 1902 | Fischer wins Nobel for sugar chemistry | Emil Fischer |
| 1928 | Griffith’s transformation experiment | Frederick Griffith |
| 1929 | Discovery of deoxyribose | Phoebus Levene |
| 1935 | First synthesis of deoxyribose | Various chemists |
| 1944 | DNA confirmed as genetic material | Avery, MacLeod, McCarty |
| 1953 | Double-helix structure of DNA proposed | Watson and Crick |
| 1954 | Deoxyribose isolated from DNA | Various researchers |
This timeline shows how understanding these sugars evolved over decades.
Biological Roles and Importance
Deoxyribose and ribose are stars in the world of nucleic acids. Deoxyribose in DNA forms the backbone for storing genetic info, with bases pairing A-T and G-C. During replication, enzymes like DNA polymerase use deoxyribonucleoside triphosphates (dNTPs) to build new strands.
Ribose in RNA comes in types like messenger RNA (mRNA), which copies DNA info; transfer RNA (tRNA), which brings amino acids; and ribosomal RNA (rRNA), part of protein-making machinery. Non-coding RNAs regulate genes.
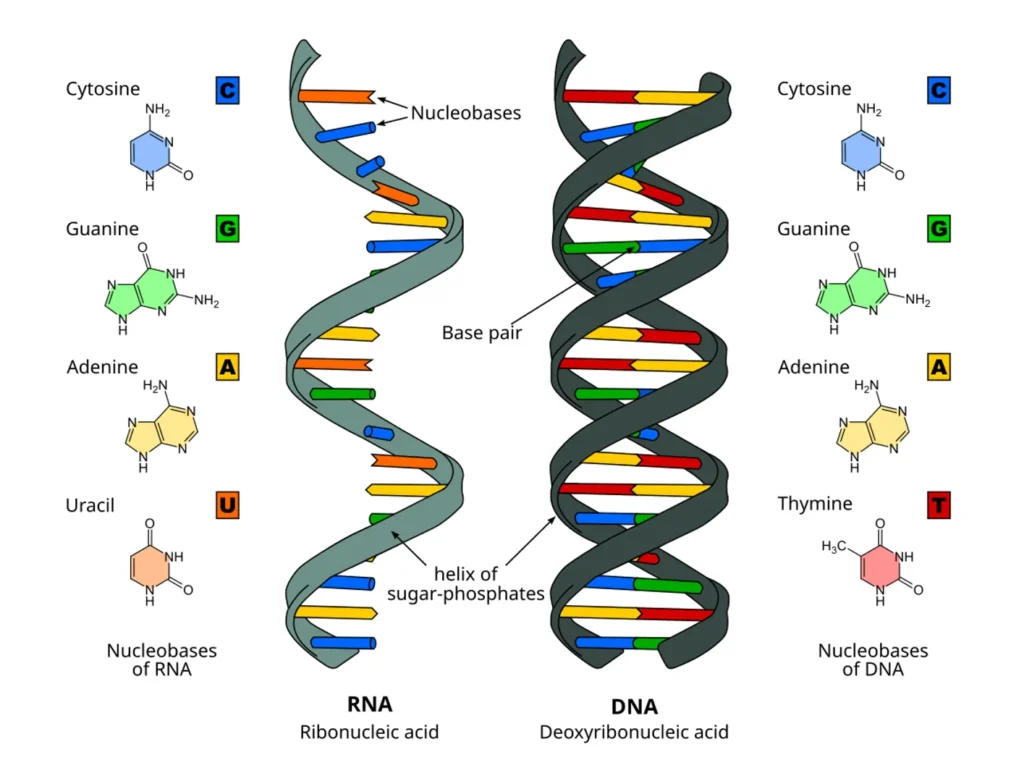
The 2′ OH in ribose makes RNA labile, suitable for temporary roles, while deoxyribose’s stability suits DNA for inheritance.
In metabolism, ribose is in the pentose phosphate pathway, generating NADPH for biosynthesis. Deoxyribose derives from ribose via ribonucleotide reductase.
Examples of Biological Importance
- Genetic Inheritance: Deoxyribose in DNA ensures traits pass accurately to offspring. Mutations here can lead to diseases like cancer.
- Protein Synthesis: Ribose in mRNA translates DNA code into proteins, like enzymes for digestion.
- Energy Transfer: Ribose in ATP powers muscle contractions and nerve signals.
- Viral Replication: Viruses like HIV use reverse transcriptase to convert RNA (ribose) to DNA (deoxyribose) for integration into host cells.
- Ribozymes: RNA molecules with ribose can act as enzymes, hinting at an “RNA world” in early life.
These examples show how these sugars underpin life’s processes.
Functions in the Human Body and Beyond
In humans, deoxyribose and ribose are synthesized endogenously. The body converts glucose to ribose via the pentose phosphate pathway, then reduces it to deoxyribose for DNA.
Deficiencies can cause issues: low ribose might affect energy levels, leading to supplements for athletes, though evidence is mixed. In diseases, altered nucleotide metabolism links to conditions like gout (purine buildup) or certain cancers.
Beyond humans, these sugars are universal in life forms, from bacteria to plants. In biotech, synthetic analogs treat viruses; for example, nucleoside analogs like acyclovir mimic deoxyribose to halt herpes replication.
In food, ribose occurs naturally in meat and is used as a sweetener or flavor enhancer.
Implications for Stability and Reactivity
The missing oxygen in deoxyribose makes DNA less prone to hydrolysis, protecting genetic info from damage. RNA’s 2′ OH allows base-catalyzed cleavage, why RNA degrades quickly.
This stability difference explains why DNA is in the nucleus, safeguarded, while RNA shuttles around the cell.
In evolution, the shift to deoxyribose might have enabled complex genomes without constant breakdown.
Advanced Insights: Synthesis and Derivatives
Ribose forms via hemiacetal reaction, closing the ring. Deoxyribose follows suit but with reduced reactivity.
Derivatives include deoxyribonucleotides like dATP, used in PCR for amplifying DNA.
In research, isotopic labeling of these sugars tracks metabolic pathways.
Comparative Table of Key Differences
To wrap up the comparisons, here’s an extensive table:
| Category | Deoxyribose | Ribose |
|---|---|---|
| Chemical Formula | C5H10O4 | C5H10O5 |
| Molar Mass | 134.13 g/mol | 150.13 g/mol |
| 2′ Position Group | H | OH |
| Found In | DNA | RNA, ATP, other molecules |
| Discovery Year | 1929 | 1891 |
| Discoverer | Phoebus Levene | Emil Fischer and Oskar Piloty |
| Stability | High, resistant to hydrolysis | Lower, more reactive |
| Biological Role | Genetic storage, inheritance | Protein synthesis, energy transfer |
| Nucleotide Types | Deoxyribonucleotides (dNTPs) | Ribonucleotides (NTPs) |
| Base Pairing | A-T, G-C | A-U, G-C (in RNA) |
| Structure in Nucleic Acid | Double helix backbone | Single strand, can fold |
| Metabolic Pathway | Derived from ribose via reduction | Synthesized in pentose phosphate pathway |
| Examples in Disease | Mutations in DNA lead to genetic disorders | RNA viruses exploit ribose for replication |
| Lab Applications | DNA sequencing, PCR | RNA extraction, in vitro transcription |
This table encapsulates the core distinctions.
Thoughts on Future Research and Applications
Looking ahead, understanding these sugars opens doors to gene therapy, where editing DNA’s deoxyribose backbone could cure diseases. RNA-based vaccines, like those for COVID-19, leverage ribose’s properties for quick immune responses.
What if we engineered hybrid sugars? Could that create more stable RNA for therapeutics? These ideas stem from decades of research and promise exciting advancements.
In education, teaching these differences helps students grasp molecular biology’s elegance. It’s a reminder that small changes, like one oxygen atom, can shape life’s complexity.
In conclusion, deoxyribose and ribose are more than just sugars; they’re the foundation of heredity and cellular function. Their differences ensure life’s diversity and continuity, making them truly remarkable molecules.
Read These Articles in Detail
- The Cell: The Fundamental Building Block of Life
- Prokaryotic Cells vs. Eukaryotic Cells: A Detailed Exploration
- Prokaryotic Cells: Definition, Anatomy, Functions, and Their Importance
- Eukaryotic Cells Explained: Definition, Structure, Functions, and Importance
- The Cell Membrane: Structure, Functions, and Evolutionary Significance
- The Cell Wall: Structure, Composition, Functions, and Evolutionary Significance
- Endomembrane System: A Detailed Exploration of the Cellular Machinery of Life
- Mitochondria: A Comprehensive Guide to Their Structure and Functions
- The Cell Envelope: The Protective Shield of Prokaryotic Cells
- Ribosomes: Definition, Structure, and Functions
- Endoplasmic Reticulum: Structure, Types, and Functions Explained
- The Golgi Apparatus: Structure, Functions, and Cellular Importance
- Cytoplasm and Nucleus: Structure, Functions, Differences, and Examples
- What Is Cell Signaling? Structure, Types, and Functions Explained
- Three Main Parts of a Nucleotide: Structure and Functions
- Enzymes: Definition, Structure, Classification, and Functions
- DNA: Structure, Function, Discovery, and Modern Applications
- DNA Replication: Steps, Process, and Genetic Importance
- Properties of DNA: Structure, Physical, and Chemical Characteristics
- DNA Packaging: How DNA Fits into the Nucleus of a Cell
- R-Factors and Plasmids: How Bacteria Develop Antibiotic Resistance
- Chromosomes: Structure, Types, Functions, and Role in Genetics
- Genetic Material: DNA, RNA, and Their Role in Heredity and Evolution
- Deoxyribose and Ribose: Structure and Function in DNA and RNA
- Differences Between Genes and DNA: Definitions, Structures, and Functions
- RNA: Definition, Structure, Types, Functions, and Its Role in Life
Frequently Asked Questions
FAQ 1: What Are Deoxyribose and Ribose, and Why Are They Important?
Deoxyribose and ribose are five-carbon sugars, or pentose sugars, that serve as critical components in the structure of nucleic acids, which store and transmit genetic information. These sugars are the backbone of DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), respectively, making them essential for life as we know it. Here’s why they matter:
- Role in Nucleic Acids: Deoxyribose forms the sugar-phosphate backbone of DNA, which carries the genetic blueprint for all living organisms. Ribose is the sugar in RNA, which helps translate that blueprint into proteins and performs other cellular tasks.
- Biological Functions: DNA, with deoxyribose, ensures stable, long-term storage of genetic information, while RNA, with ribose, is involved in short-term processes like protein synthesis and gene regulation.
- Energy Transfer: Ribose is also a key component of ATP (adenosine triphosphate), the molecule that powers cellular activities like muscle movement and nerve signaling.
- Universal Presence: Both sugars are found in all life forms, from bacteria to humans, highlighting their fundamental role in biology.
Their slight structural differences lead to significant functional distinctions, which we’ll explore further in other FAQs.
FAQ 2: How Do the Chemical Structures of Deoxyribose and Ribose Differ?
The chemical structures of deoxyribose and ribose are very similar, but a small difference at one carbon atom sets them apart. Here’s a breakdown:
- Chemical Formula: Deoxyribose has the formula C5H10O4, while ribose is C5H10O5, indicating ribose has one extra oxygen atom.
- Key Structural Difference: At the 2′ carbon position, deoxyribose has a hydrogen (H) atom, whereas ribose has a hydroxyl (OH) group. This missing oxygen in deoxyribose is why it’s called “deoxy” (without oxygen).
- Ring Structure: Both form a five-membered furanose ring in their cyclic form, common in nucleic acids, but the 2′ OH group in ribose makes it more reactive.
- Impact on Function: The absence of the 2′ OH in deoxyribose makes DNA more stable, ideal for long-term genetic storage. Ribose’s extra OH allows RNA to fold into complex shapes for roles like protein synthesis.
This subtle difference is crucial for how DNA and RNA function in cells.
FAQ 3: Why Does DNA Use Deoxyribose Instead of Ribose?
DNA uses deoxyribose instead of ribose because it provides greater chemical stability, which is essential for its role as the long-term storage of genetic information. Here’s why:
- Stability Advantage: The absence of the hydroxyl group at the 2′ carbon in deoxyribose makes DNA less prone to hydrolysis (chemical breakdown by water). This stability protects genetic information from damage over time.
- Double-Helix Structure: Deoxyribose contributes to DNA’s ability to form a stable double helix, where two strands pair up via hydrogen bonds between bases like adenine and thymine. This structure is ideal for replication and storage.
- Enzymatic Recognition: The lack of the 2′ OH group helps enzymes distinguish DNA from RNA, preventing errors in processes like replication or transcription.
- Evolutionary Benefit: The stability of deoxyribose likely evolved to support complex genomes in organisms, ensuring accurate inheritance of traits across generations.
In contrast, ribose’s reactivity suits RNA’s temporary roles, like carrying genetic messages.
FAQ 4: What Role Does Ribose Play in RNA and Other Molecules?
Ribose is a versatile sugar that plays a central role in RNA and other critical molecules in the body. Here’s how it functions:
- RNA Backbone: Ribose forms the sugar-phosphate backbone of RNA, which includes types like messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA). These RNAs are key in protein synthesis and gene regulation.
- Energy Molecules: Ribose is a component of ATP, the cell’s energy currency, which powers processes like muscle contraction and biochemical reactions.
- Coenzymes: Ribose is found in molecules like NADH and FADH2, which are involved in cellular respiration and energy production.
- Flexibility in RNA: The 2′ hydroxyl group in ribose allows RNA to form complex shapes, like loops and hairpins, enabling functions such as catalysis in ribozymes (RNA enzymes).
For example, during protein synthesis, mRNA (containing ribose) carries genetic instructions from DNA to ribosomes, where proteins are built.
FAQ 5: How Were Deoxyribose and Ribose Discovered?
The discovery of deoxyribose and ribose is tied to the history of nucleic acid research. Here’s a look at their origins:
- Ribose Discovery (1891): Chemists Emil Fischer and Oskar Piloty identified ribose while studying carbohydrates. Fischer’s work on sugars was so groundbreaking that he won the Nobel Prize in Chemistry in 1902.
- Deoxyribose Discovery (1929): Phoebus Levene discovered deoxyribose while analyzing the components of DNA. It was later synthesized in 1935 and isolated from DNA in 1954.
- Broader Context: The study of nucleic acids began with Friedrich Miescher’s discovery of “nuclein” in 1869, followed by Albrecht Kossel’s work on DNA components. The double-helix structure of DNA, proposed by Watson and Crick in 1953, further highlighted deoxyribose’s role.
- Impact: These discoveries laid the foundation for modern genetics, enabling advancements like DNA sequencing and gene editing.
The timeline of these findings shows how our understanding of these sugars evolved over decades.
FAQ 6: How Do Deoxyribose and Ribose Affect the Stability of DNA and RNA?
The stability of DNA and RNA is heavily influenced by their respective sugars, deoxyribose and ribose. Here’s how:
- Deoxyribose in DNA: The absence of the 2′ hydroxyl group in deoxyribose makes DNA resistant to hydrolysis, a process where water molecules break chemical bonds. This stability allows DNA to store genetic information safely in the cell nucleus for years or even a lifetime.
- Ribose in RNA: The 2′ OH group in ribose makes RNA more reactive and prone to breaking down. This lability suits RNA’s role in temporary tasks, like carrying genetic messages or forming part of ribosomes.
- Functional Implications: DNA’s stability supports its role in long-term genetic storage, while RNA’s instability allows it to be quickly synthesized and degraded as needed.
- Example: DNA remains intact during cell division, ensuring accurate copying, while RNA’s short lifespan is ideal for rapid responses, like producing proteins during a viral infection.
This difference ensures that DNA and RNA fulfill their distinct roles effectively.
FAQ 7: What Are the Biological Implications of Deoxyribose and Ribose?
Deoxyribose and ribose have profound biological implications due to their roles in nucleic acids and beyond. Here’s a detailed look:
- Genetic Storage and Expression: Deoxyribose in DNA ensures accurate storage and replication of genetic information, critical for traits like eye color or disease resistance. Ribose in RNA enables gene expression, translating DNA’s code into proteins like insulin.
- Energy Metabolism: Ribose is part of ATP and coenzymes like NADH, which are vital for energy production in cells. For instance, ATP powers muscle contractions during exercise.
- Cellular Regulation: Non-coding RNAs, containing ribose, regulate gene expression, influencing processes like cell growth or stress responses.
- Disease and Medicine: Mutations in DNA’s deoxyribose backbone can lead to diseases like cancer, while RNA-based therapies (e.g., mRNA vaccines) leverage ribose for immune responses.
Their differences ensure that DNA and RNA work together seamlessly to support life.
FAQ 8: Can Deoxyribose and Ribose Be Synthesized in the Body?
Yes, the human body can synthesize both deoxyribose and ribose through metabolic pathways. Here’s how it works:
- Ribose Synthesis: Ribose is produced via the pentose phosphate pathway, a metabolic process that converts glucose into ribose-5-phosphate. This pathway also generates NADPH, used in biosynthesis.
- Deoxyribose Synthesis: Deoxyribose is derived from ribose through the action of the enzyme ribonucleotide reductase, which removes the 2′ hydroxyl group from ribose-containing nucleotides to form deoxyribonucleotides.
- Biological Importance: These pathways ensure a steady supply of sugars for DNA replication, RNA synthesis, and energy molecules like ATP.
- Supplements: While the body makes enough ribose for most needs, some people take ribose supplements to boost energy, especially athletes, though scientific evidence on their effectiveness is mixed.
These processes highlight the body’s ability to produce these sugars naturally.
FAQ 9: How Are Deoxyribose and Ribose Used in Biotechnology and Medicine?
Deoxyribose and ribose are pivotal in biotechnology and medical applications due to their roles in nucleic acids. Here’s how they’re utilized:
- DNA Sequencing and PCR: Deoxyribose-containing nucleotides (dNTPs) are used in polymerase chain reaction (PCR) to amplify DNA for genetic testing, such as identifying mutations in cancer genes.
- RNA-Based Therapies: Ribose is part of mRNA vaccines, like those for COVID-19, which instruct cells to produce proteins that trigger an immune response.
- Antiviral Drugs: Nucleoside analogs, mimicking deoxyribose or ribose, are used in drugs like acyclovir to block viral replication in diseases like herpes.
- Research Tools: Isotopically labeled deoxyribose and ribose help scientists track metabolic pathways, aiding research into diseases like diabetes or cancer.
These applications show how these sugars drive cutting-edge medical advancements.
FAQ 10: What Would Happen If DNA Used Ribose Instead of Deoxyribose?
If DNA used ribose instead of deoxyribose, it would have significant consequences for genetic stability and cellular function. Here’s what could happen:
- Reduced Stability: Ribose’s 2′ hydroxyl group makes RNA prone to hydrolysis, so DNA with ribose would break down more easily, risking loss of genetic information.
- Altered Structure: The extra OH group might prevent DNA from forming its stable double-helix structure, disrupting replication and base pairing.
- Enzymatic Confusion: Enzymes that distinguish DNA from RNA based on the 2′ position might malfunction, leading to errors in replication or transcription.
- Evolutionary Impact: The instability could have limited the complexity of genomes, making it harder for organisms to evolve into complex forms like humans.
This hypothetical scenario underscores why deoxyribose is perfectly suited for DNA’s role in long-term genetic storage.
FAQ 11: What Are the Key Chemical Properties of Deoxyribose and Ribose?
Deoxyribose and ribose share many chemical traits as pentose sugars, but their properties differ in ways that influence their biological roles. Both are aldopentoses, featuring an aldehyde group in their open-chain form, and they typically exist in a furanose ring structure within nucleic acids. However, ribose’s extra hydroxyl group affects its polarity and reactivity, making it more hydrophilic than deoxyribose. These properties are crucial for how they interact with enzymes and other molecules in cells.
In terms of solubility, both dissolve well in water due to their multiple hydroxyl groups, but ribose’s additional OH enhances this slightly, aiding its role in dynamic cellular processes. Their optical activity also stems from chiral centers, with both commonly found in the D-configuration in nature. Understanding these properties helps explain why deoxyribose contributes to DNA’s rigidity, while ribose allows RNA to be more flexible.
| Property | Deoxyribose | Ribose |
|---|---|---|
| Chemical Formula | C5H10O4 | C5H10O5 |
| Molar Mass | 134.13 g/mol | 150.13 g/mol |
| Melting Point | Around 91°C | Approximately 95°C |
| Solubility in Water | Highly soluble, but slightly less than ribose | Extremely soluble due to extra OH |
| Reactivity | Less reactive, stable against hydrolysis | More reactive, prone to base-catalyzed cleavage |
| Optical Rotation | D-form is dextrorotatory | D-form is levorotatory |
| pH Stability | Stable in neutral to slightly acidic conditions | Similar, but 2′ OH affects alkaline sensitivity |
This table illustrates how small molecular tweaks lead to distinct chemical behaviors.
FAQ 12: How Do Deoxyribose and Ribose Undergo Phosphorylation in Cells?
Phosphorylation is a key process where deoxyribose and ribose are modified to form nucleotides, the building blocks of DNA and RNA. For ribose, this typically starts in the pentose phosphate pathway, where glucose is converted into ribose-5-phosphate. Enzymes like ribokinase then add a phosphate group to the 5′ carbon, creating ribose-5-phosphate, which can further react with nitrogenous bases to form ribonucleotides like AMP or GMP. This step is energy-dependent, often using ATP, and it’s essential for RNA synthesis and energy molecules.
Deoxyribose phosphorylation follows a different route since the body doesn’t make deoxyribose directly; instead, it’s derived from ribose nucleotides. The enzyme ribonucleotide reductase converts ribonucleoside diphosphates (like ADP) into deoxyribonucleoside diphosphates (dADP) by replacing the 2′ OH with H. Then, kinases add another phosphate to form triphosphates like dATP, ready for DNA polymerase during replication. This process is tightly regulated to balance nucleotide pools, preventing mutations.
Both sugars’ phosphorylation ensures efficient nucleic acid assembly, but deoxyribose’s pathway includes an extra reduction step, highlighting DNA’s need for stability. Disruptions here can lead to cellular imbalances, underscoring the precision of these biochemical mechanisms.
FAQ 13: Why Did Evolution Favor Deoxyribose for DNA and Ribose for RNA?
Evolution likely selected deoxyribose for DNA because its lack of the 2′ hydroxyl group provides greater chemical stability, reducing the risk of spontaneous hydrolysis that could damage genetic information. In early life forms, an “RNA world” may have dominated, where ribose-based RNA handled both storage and catalysis. However, as organisms grew more complex, the need for a more durable molecule arose to store larger genomes without constant degradation.
Transitioning to deoxyribose allowed DNA to serve as a reliable archive, while ribose’s reactivity kept RNA suited for transient roles like transcription and translation. This division of labor probably emerged through natural selection, favoring cells that could maintain genetic fidelity over generations. Fossil evidence and biochemical models suggest this shift occurred around 3.5 billion years ago, enabling the rise of diverse life.
Today, this evolutionary choice underpins modern biology, from bacterial replication to human heredity, showing how a single atomic difference shaped life’s complexity.
FAQ 14: Are There Health Benefits to Ribose Supplements?
Ribose supplements are often marketed for boosting energy and athletic performance, but their benefits are debated.
- Energy Boost: Ribose helps form ATP, so supplements might aid recovery after intense exercise by replenishing nucleotide pools in muscles. Some studies suggest they reduce fatigue in conditions like fibromyalgia.
- Heart Health: In people with heart disease, ribose could improve cardiac function by enhancing energy metabolism in heart cells, potentially easing symptoms of ischemia.
- Athletic Use: Endurance athletes sometimes use it to speed up recovery, though evidence is mixed; it may help in high-intensity training but not casual workouts.
- Potential Drawbacks: High doses can cause side effects like gastrointestinal upset, and it’s not recommended for everyone without medical advice.
- Scientific View: While the body makes ribose naturally, supplements might benefit those with deficiencies, but more research is needed for broad claims.
Always consult a doctor before starting supplements.
FAQ 15: How Are Deoxyribose and Ribose Synthesized in Laboratories?
Lab synthesis of these sugars allows for research and pharmaceutical production.
- Ribose Synthesis: Often starts with glucose or other carbohydrates using the Fischer-Kiliani synthesis, involving chain elongation and reduction steps to form the pentose structure. Enzymatic methods mimic natural pathways for higher purity.
- Deoxyribose Synthesis: Typically derived from ribose by selective deoxygenation at the 2′ position using reagents like tributyltin hydride or through microbial fermentation.
- Modern Techniques: Biotechnology employs genetically modified bacteria to produce these sugars efficiently, reducing costs for drug development.
- Applications: Synthesized versions are used in antiviral drugs and genetic studies, ensuring consistent supply.
- Challenges: Achieving stereospecificity is key to match natural D-forms, avoiding inactive isomers.
These methods have evolved since early discoveries, supporting advances in molecular biology.
FAQ 16: What Methods Are Used to Detect Deoxyribose and Ribose in Samples?
Detecting these sugars is vital in biochemistry labs for analyzing nucleic acids.
| Detection Method | Description | Applications |
|---|---|---|
| Chromatography | Techniques like HPLC separate sugars based on polarity; deoxyribose elutes differently due to less OH groups. | Quantifying in DNA/RNA extracts or biological fluids. |
| Spectroscopy | NMR or IR identifies structures by unique peaks; ribose shows extra signals from 2′ OH. | Structural confirmation in research samples. |
| Colorimetric Assays | Diphenylamine test turns blue with deoxyribose in DNA, while orcinol reacts with ribose in RNA. | Quick estimation in tissue or cell lysates. |
| Enzymatic Methods | Specific enzymes like ribose-5-phosphate isomerase detect ribose through coupled reactions. | Precise measurement in metabolic studies. |
| Mass Spectrometry | MS/MS distinguishes by mass differences (134 vs 150 Da), often after derivatization. | High-sensitivity analysis in complex mixtures. |
These tools ensure accurate identification in various contexts.
FAQ 17: How Do Deoxyribose and Ribose Function in Non-Human Organisms?
In bacteria, deoxyribose forms the backbone of DNA just as in humans, enabling rapid replication crucial for survival in changing environments. Ribose, meanwhile, is integral to RNA for protein production and even in riboswitches that sense metabolites and regulate genes. Plants use these sugars similarly, with deoxyribose in chloroplast DNA for photosynthesis genes and ribose in RNA for growth responses.
Viruses exploit host machinery; DNA viruses like herpes incorporate deoxyribose, while RNA viruses like influenza rely on ribose for their genomes. In archaea, adapted to extreme conditions, these sugars maintain genetic stability despite heat or acidity.
Across kingdoms, the sugars’ roles underscore universal principles of life, adapted to diverse niches.
FAQ 18: What Diseases Are Linked to Deoxyribose and Ribose Metabolism?
Imbalances in these sugars’ metabolism can contribute to various health issues.
- Nucleotide Deficiency Disorders: Defects in ribonucleotide reductase, which converts ribose to deoxyribose forms, lead to immunodeficiencies like severe combined immunodeficiency (SCID).
- Cancer Connections: Altered deoxyribose nucleotide pools fuel rapid DNA replication in tumors, making enzymes targets for chemotherapy drugs.
- Metabolic Syndromes: Pentose phosphate pathway disruptions affect ribose production, linking to conditions like diabetes where energy metabolism falters.
- Neurological Issues: Ribose shortages in ATP synthesis may play a role in chronic fatigue syndrome or neurodegenerative diseases.
- Genetic Mutations: Errors in DNA’s deoxyribose backbone cause hereditary diseases like sickle cell anemia.
Research continues to uncover these links for better treatments.
FAQ 19: What Future Research Directions Involve Deoxyribose and Ribose?
Future studies on deoxyribose and ribose promise breakthroughs in synthetic biology and medicine. Scientists are exploring ways to engineer hybrid nucleic acids, combining ribose’s flexibility with deoxyribose’s stability, to create more robust gene therapies for conditions like cystic fibrosis. This could lead to longer-lasting RNA drugs that resist degradation.
In evolutionary biology, researchers aim to recreate prebiotic conditions to understand how ribose formed in primordial soups, shedding light on life’s origins. Advances in nanotechnology might use these sugars in biosensors for early disease detection.
With CRISPR and other tools, modifying sugar metabolism could combat antibiotic resistance in bacteria or enhance crop resilience in plants. Overall, these directions highlight the sugars’ potential in addressing global challenges.
FAQ 20: How Do Deoxyribose and Ribose Compare in Terms of Evolutionary and Metabolic Aspects?
| Aspect | Deoxyribose | Ribose |
|---|---|---|
| Evolutionary Origin | Likely evolved from ribose reduction for stability in complex genomes. | Predates deoxyribose; central in RNA world hypothesis. |
| Metabolic Synthesis | Derived from ribose via ribonucleotide reductase. | Produced in pentose phosphate pathway from glucose. |
| Energy Cost | Higher, due to additional enzymatic steps. | Lower, directly from carbohydrate metabolism. |
| Role in Primitive Life | Absent in early RNA-based systems. | Enabled self-replicating RNA molecules. |
| Modern Adaptations | Supports large, stable genomes in eukaryotes. | Versatile in regulatory RNAs across all life. |
| Disease Implications | Imbalances lead to DNA repair defects. | Deficiencies affect ATP and energy levels. |
This comparison reveals their intertwined histories and functions.
Acknowledgement
The Examsmeta.com website expresses its sincere gratitude to the following reputable sources for providing valuable insights and reliable information that enriched the content of the article “Deoxyribose vs Ribose: Unraveling the Crucial Differences in DNA and RNA Sugars.” Their comprehensive resources on molecular biology, biochemistry, and nucleic acid research were instrumental in ensuring the accuracy and depth of this article.
Below is a list of the key sources that contributed to our understanding:
- National Center for Biotechnology Information (NCBI): For detailed biochemical data and research on nucleic acids.
- Khan Academy: For clear, educational explanations of DNA and RNA structures.
- Encyclopaedia Britannica: For historical context and foundational knowledge on sugars and nucleic acids.
- PubChem: For precise chemical properties and structural information on deoxyribose and ribose.
- Nature Education: For insights into evolutionary biology and molecular mechanisms.

