Antibiotic resistance has become one of the most pressing challenges in modern medicine, threatening to undo decades of progress in treating bacterial infections. At the heart of this issue lie small, circular pieces of DNA known as plasmids, which allow bacteria to share genetic traits rapidly and efficiently. Among these, R-factors, or resistance plasmids, play a starring role by carrying genes that help bacteria withstand antibiotics and even toxic metals. Discovered over half a century ago, these genetic elements highlight how bacteria evolve and adapt in ways that outpace our medical defenses.
In this comprehensive article, we’ll dive deep into what plasmids are, their various types, the specifics of R-factors, their structures and functions, and the broader implications for health and the environment. We’ll explore real-world examples, historical context, and even some forward-thinking ideas on how we might combat this growing threat. Whether you’re a student, researcher, or just curious about microbiology, this guide aims to break it down in simple, everyday language while packing in plenty of details.
Table of Contents
What Are Plasmids?
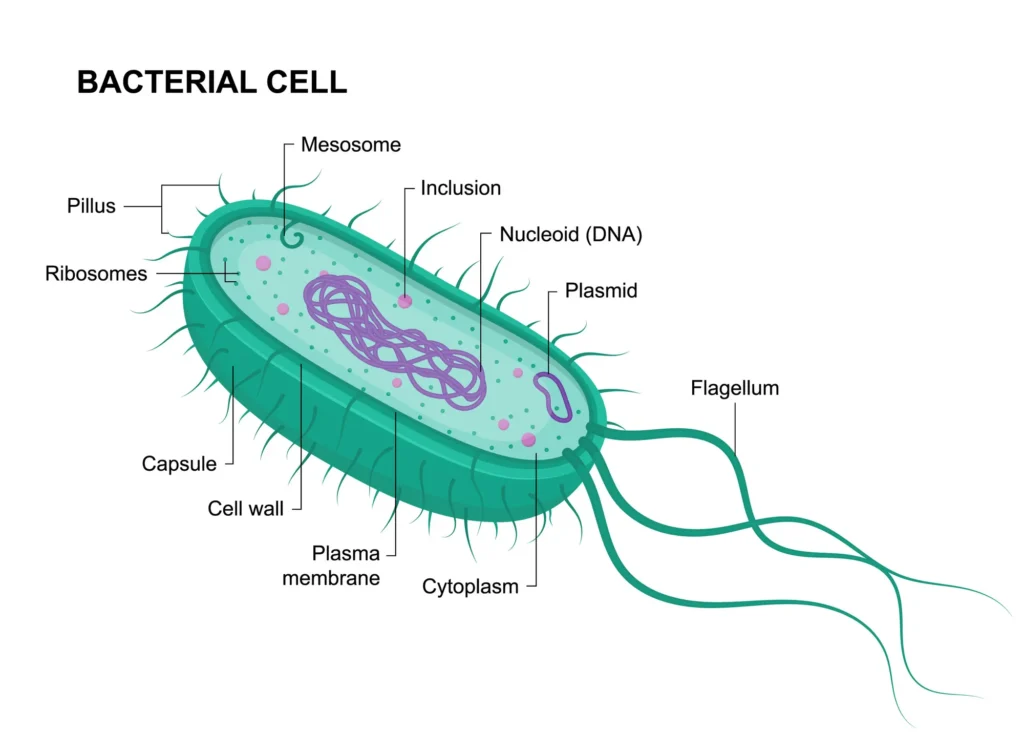
Plasmids are essentially bonus packets of genetic information that bacteria carry outside their main chromosome. Unlike the bacterial chromosome, which holds the core instructions for survival and reproduction, plasmids are smaller, circular DNA molecules that can replicate on their own. This independence makes them incredibly versatile, allowing bacteria to pick up new abilities without altering their primary genome.
Imagine a bacterium as a basic smartphone: the chromosome is the operating system, essential but fixed. Plasmids are like apps you can download, install, and even share with other phones. They range in size from a few thousand base pairs to over a hundred thousand, and while not every bacterium has them, they’re common in many strains across bacteria, archaea, and even some eukaryotic organisms like yeast or plants.
One key feature of plasmids is their ability to be transferred between bacteria through processes like conjugation, transformation, or transduction. This horizontal gene transfer lets beneficial traits spread quickly through a population, much like how viral memes explode online. In natural environments, plasmids help bacteria survive harsh conditions, degrade pollutants, or infect hosts. But in the context of human health, they often spell trouble by spreading antibiotic resistance.
To give you a sense of their prevalence, studies show that in environments like soil, water, or the human gut, up to 20 percent of bacterial genes might come from plasmids or other mobile elements. This genetic mobility drives bacterial evolution at a rapid pace, far faster than mutations alone could achieve.
- Key Properties of Plasmids: They are extrachromosomal, meaning they’re not part of the main DNA strand. They’re typically circular, though linear plasmids exist in some bacteria like those in the Actinobacteria group.
- Replication Independence: Plasmids have their own origin of replication, allowing them to multiply separately from the chromosome.
- Copy Number Variability: Some plasmids exist in low copy numbers (one or two per cell), while others can have dozens, influencing how strongly their traits are expressed.
- Host Range: Broad-host-range plasmids can jump between different bacterial species, while narrow ones stick to closely related hosts.
For example, in Escherichia coli, a common gut bacterium, plasmids can carry genes for lactose metabolism, helping the bacteria digest milk sugars in dairy-rich environments.
Exploring the Diverse Types of Plasmids in Bacteria
Bacteria harbor a variety of plasmid types, each tailored to specific functions that enhance survival, reproduction, or adaptation. These categories aren’t rigid; some plasmids combine traits from multiple types, creating hybrid elements that are even more potent. Let’s break them down with details and examples to illustrate their roles.
Fertility Plasmids (F-Plasmids or Sex Factor Plasmids)
These are the matchmakers of the bacterial world. F-plasmids contain genes for building a sex pilus, a tube-like structure that connects donor (F+) and recipient (F-) cells during conjugation. This process transfers DNA, including the plasmid itself, turning the recipient into a donor.
In practical terms, F-plasmids facilitate the spread of other genetic material. For instance, in E. coli, the classic F-plasmid enables rapid gene sharing in dense populations like the intestines.
- Donor vs. Recipient Dynamics: F+ cells act as males, donating DNA, while F- cells receive it.
- Integration Potential: Some F-plasmids can insert into the chromosome, forming Hfr (high-frequency recombination) strains that transfer large chromosomal chunks.
Resistance Plasmids (R-Plasmids or R-Factors)
The stars of antibiotic resistance stories, R-plasmids carry genes that protect bacteria from drugs like tetracycline, streptomycin, and ampicillin, as well as metals such as mercury or cadmium. They’re conjugative, meaning they can transfer themselves, amplifying resistance across populations.
A real-world example is the NR1 plasmid in Shigella, which confers multi-drug resistance and has been studied for its modular structure.
- Metal Resistance Overlap: Many R-plasmids also handle heavy metals, useful in polluted environments.
- Clinical Impact: In hospitals, these plasmids fuel outbreaks of resistant strains like methicillin-resistant Staphylococcus aureus (MRSA).
Colicin Plasmids (Col-Plasmids)
These plasmids arm bacteria with weapons: genes for producing colicins, toxic proteins that kill competing bacteria. It’s like bacterial chemical warfare, securing resources in crowded niches.
For instance, ColE1 in E. coli produces a toxin that punctures rival cell membranes, giving the host a competitive edge.
- Immunity Genes: The plasmid also codes for proteins that protect the host from its own toxin.
- Narrow Specificity: Colicins target specific receptors, making them selective assassins.
Degradative or Catabolic Plasmids
These environmental heroes (or villains, depending on context) enable bacteria to break down complex compounds like hydrocarbons, pesticides, or aromatic chemicals. They’re crucial in bioremediation, cleaning up oil spills or industrial waste.
The TOL plasmid in Pseudomonas putida degrades toluene, a pollutant from gasoline, turning it into harmless byproducts.
- Metabolic Pathways: They encode entire enzyme cascades for catabolism.
- Applications in Biotech: Engineers use these for eco-friendly waste treatment.
Virulence Plasmids
Virulence plasmids turn harmless bacteria into pathogens by providing genes for toxins, adhesins, or invasion factors. They’re key in diseases like anthrax or plague.
In Yersinia pestis, the pPCP1 plasmid codes for plasminogen activator, aiding tissue invasion during bubonic plague.
- Toxin Production: Examples include enterotoxins in E. coli causing diarrhea.
- Host Interaction: Genes for pili help bacteria stick to host cells.
Heavy Metal Resistance Plasmids
Specialized for toxic environments, these plasmids detoxify metals like arsenic, cadmium, or mercury through efflux pumps or chemical modification.
The pMER327 in Bacillus cereus resists mercury by converting it to volatile forms.
- Environmental Adaptation: Common in mining sites or polluted waters.
- Co-Selection with Antibiotics: Metals can indirectly promote antibiotic resistance by favoring plasmid-carrying bacteria.
To organize this further, here’s a detailed table comparing the main types of plasmids:
| Plasmid Type | Primary Function | Key Genes/Traits | Examples | Host Bacteria | Transfer Mechanism | Real-World Relevance |
|---|---|---|---|---|---|---|
| Fertility (F-Plasmids) | Facilitate conjugation and gene transfer | tra genes for pilus formation | F-plasmid in E. coli | Escherichia coli, other Enterobacteriaceae | Conjugation | Spreads resistance in gut microbiomes |
| Resistance (R-Plasmids) | Confer resistance to antibiotics and metals | tetA for tetracycline, mer for mercury | NR1 in Shigella, IncFII plasmids | Shigella, Klebsiella, Salmonella | Conjugation, transformation | Drives hospital superbugs like CRE (carbapenem-resistant Enterobacteriaceae) |
| Colicin (Col-Plasmids) | Produce bacteriocins to kill competitors | col genes for toxin and immunity | ColE1, ColIb | E. coli, other coliforms | Often non-conjugative, but can hitchhike | Maintains dominance in biofilms |
| Degradative (Catabolic) | Break down xenobiotics and pollutants | Enzyme pathways like TOL for toluene | TOL plasmid, CAM for camphor | Pseudomonas spp., Rhodococcus | Conjugation in some | Bioremediation of oil spills, e.g., Exxon Valdez cleanup |
| Virulence | Enhance pathogenicity | Toxin genes, adhesins | pPCP1 in Yersinia, Ti plasmid in Agrobacterium (plant pathogen) | Yersinia pestis, Salmonella enterica | Conjugation or stable maintenance | Causes diseases like plague or food poisoning |
| Heavy Metal Resistance | Detoxify metals | Efflux pumps, reductases | pMOL30 for multiple metals | Alcaligenes eutrophus, Pseudomonas | Often conjugative | Survives in industrial waste, co-selects for antibiotic resistance |
This table shows how plasmids overlap in functions, with many resistance plasmids also carrying virulence or metal tolerance genes, creating multi-threat elements.
The History of R-Factors
The story of R-factors begins in post-war Japan, amid a dysentery epidemic caused by Shigella strains. In 1959, researchers noticed these bacteria had suddenly become resistant to multiple antibiotics like sulfonamides, streptomycin, and tetracycline, which had been effective before. This wasn’t due to mutations but transferable factors, dubbed R-factors (for resistance).
By the 1960s, scientists like Tsutomu Watanabe confirmed these were plasmids, separate from the chromosome, and capable of conjugation. The discovery paralleled the F-plasmid work, revealing structural similarities. As antibiotic use surged in medicine and agriculture, R-factors spread globally, evolving into multi-drug resistance carriers.
Today, with genomics, we see R-factors in diverse bacteria, from hospital pathogens to environmental microbes. Their history underscores a key idea: human activities accelerate natural evolutionary processes, turning rare traits into widespread problems.
- Milestones in Discovery:
- 1950s: Initial observations in Japan during Shigella outbreaks.
- 1960s: Confirmation as plasmids; mapping of resistance genes.
- 1970s: Detailed structure analysis, identifying RTF and R-determinants.
- 1980s-2000s: Link to transposons and integrons for gene mobility.
- Present: Genomic sequencing reveals ancient origins, predating human antibiotics.
This timeline shows how scientific understanding evolved alongside the resistance crisis.
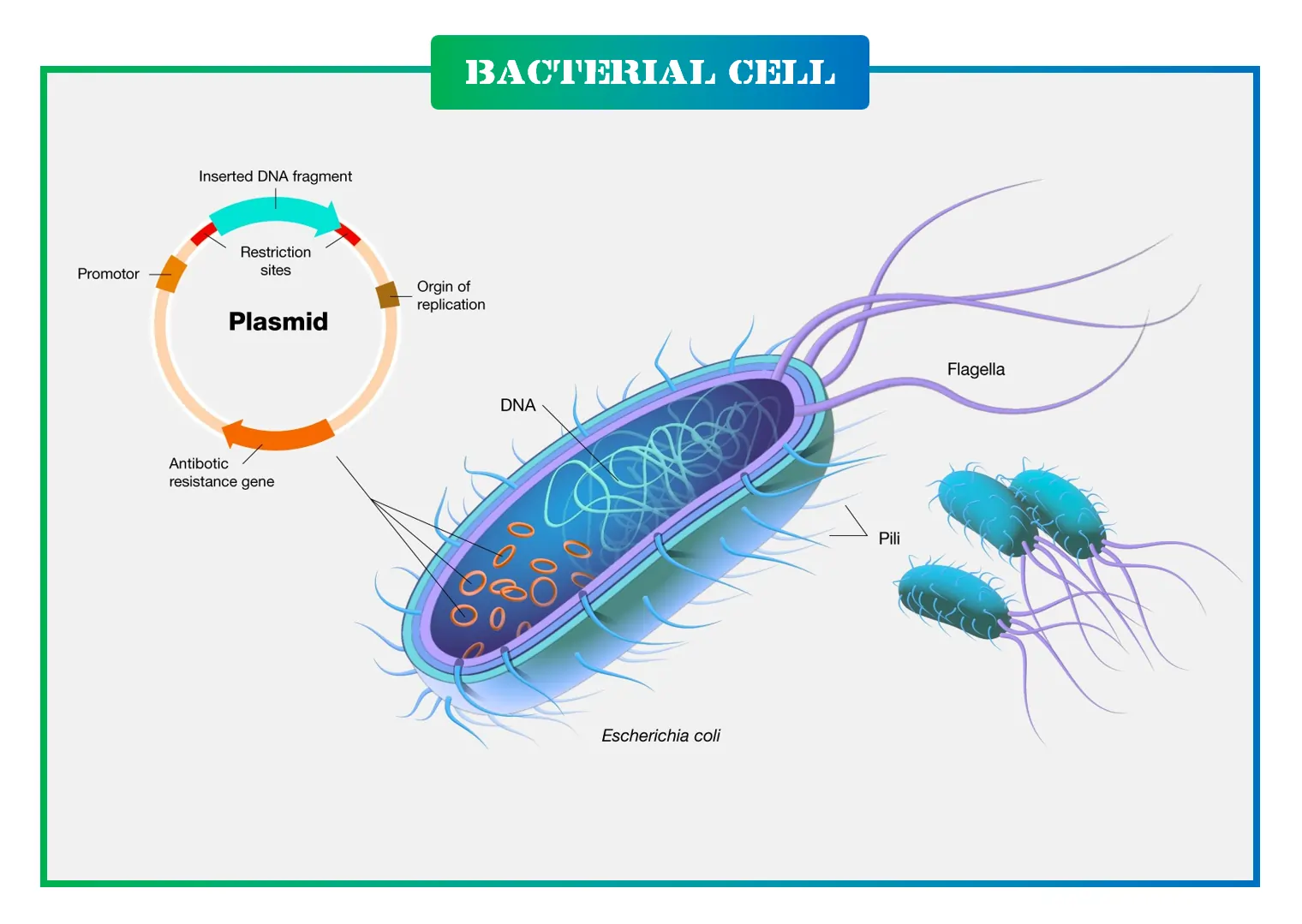
Delving into the Structure of Resistance Plasmids
R-plasmids are marvels of genetic engineering, typically 80 to 95 kilobases long, forming circular DNA loops. They’re composed of two main parts: the Resistance Transfer Factor (RTF) and the R-determinant.
The RTF handles replication, conjugation, and stability, similar to F-plasmids, with genes like tra for transfer and finO for repression. It makes up the bulk, ensuring the plasmid’s survival and spread.
The R-determinant is smaller, carrying the resistance genes, flanked by insertion sequences (IS1 elements) that allow gene swapping. This modularity lets R-plasmids evolve by acquiring new resistances via transposons or integrons.
In some cases, R-plasmids integrate into chromosomes or form cointegrates, blending traits.
- Structural Components:
- Origin of Replication (oriV): Ensures independent copying.
- Transfer Operon: Genes for pilus and conjugation machinery.
- Resistance Cassettes: Variable regions with genes like bla for beta-lactamases.
- Insertion Sequences: Mobile elements facilitating rearrangements.
For visualization, consider this table of common structural elements in R-plasmids:
| Component | Description | Size Range | Role in Function | Examples of Genes |
|---|---|---|---|---|
| RTF (Resistance Transfer Factor) | Core for transfer and replication | 50-70 kb | Enables conjugation and stability | traA-traZ, repA |
| R-Determinant | Resistance gene cluster | 10-30 kb | Confers specific resistances | tetR (tetracycline), aadA (aminoglycosides) |
| IS Elements (e.g., IS1) | Short mobile sequences | 0.7-1.5 kb | Gene insertion and excision | IS1, IS26 |
| Transposons | Larger mobile units | 5-20 kb | Carry multiple genes, jump between plasmids | Tn3 (ampicillin), Tn5 (kanamycin) |
| Integrons | Gene capture systems | Variable | Acquire new cassettes via integrase | intI1, with sul1 (sulfonamide) |
| Partitioning System | Ensures distribution during division | 2-5 kb | Maintains plasmid in daughter cells | parA/parB |
This setup allows R-plasmids to be highly adaptable, like Lego blocks for resistance.
Key Functions of Resistance Plasmids
R-plasmids do more than resist antibiotics; they orchestrate bacterial adaptation on multiple fronts. Primarily, they encode enzymes that inactivate drugs, pump them out, or alter targets.
They also promote pili formation, aiding biofilm creation and conjugation.
In gene amplification, low drug doses trigger resistance gene duplication, boosting resistance levels through recombination during replication.
Horizontal gene transfer is their superpower: via conjugation, transformation, or phage-mediated transduction, resistance spreads rapidly.
- Transmission of MDR Genes: R-plasmids shuttle multi-drug resistance (MDR) across species.
- Autonomous Replication and Conjugation: They self-replicate and transfer independently.
- Pili Production: Enhances attachment and gene exchange.
Thoughts on this: In a world without antibiotics, these functions might seem benign, but our overuse has weaponized them.
Gene Amplification: How R-Plasmids Ramp Up Resistance
When bacteria face sub-lethal antibiotic doses, survivors often show heightened resistance due to gene amplification in the R-determinant. This involves duplicating resistance genes during replication, sometimes creating tandem arrays.
For example, in cells with tet genes, low tetracycline leads to multiple copies, increasing efflux pumps.
This process is reversible; without pressure, copies diminish, but it buys time for permanent mutations.
- Mechanism Steps:
- Exposure to low concentrations.
- Recombination between replicating strands.
- Amplification of specific genes.
- Increased expression for survival.
Real-world implication: Sub-therapeutic antibiotics in farming accelerate this, contributing to resistance reservoirs.
Horizontal Gene Transfer
Horizontal gene transfer (HGT) via R-plasmids is like bacterial file-sharing. Conjugation, the main route, uses the sex pilus to pass DNA. Many R-plasmids include F-like elements, enhancing this.
Transformation involves uptake of free DNA, while transduction uses viruses.
In environments like wastewater, HGT flourishes, spreading resistance from harmless to pathogenic bacteria.
- Factors Influencing HGT:
- Cell density: Crowded settings boost conjugation.
- Environmental stress: Pollutants or antibiotics promote transfer.
- Plasmid Compatibility: Determines if multiple plasmids coexist.
Idea: Targeting conjugation proteins could be a novel anti-resistance strategy.
Real-World Examples of Plasmid-Mediated Resistance Spread
Plasmids have fueled numerous outbreaks. In the 2011 E. coli O104:H4 epidemic in Europe, a virulence plasmid carried Shiga toxin genes, causing hemolytic uremic syndrome.
In hospitals, IncF plasmids in Klebsiella pneumoniae spread carbapenem resistance (blaKPC), leading to untreatable pneumonia.
Environmental cases: In rivers near pharmaceutical plants, plasmids carry mcr-1 for colistin resistance, jumping to human pathogens.
- Case Studies:
- NDM-1 Superbug: Plasmid-borne metallo-beta-lactamase gene spread from India globally, resisting most beta-lactams.
- Vibrio cholerae in Haiti: Post-earthquake, plasmids transferred toxin genes, worsening cholera.
- Salmonella in Poultry: R-plasmids confer resistance to fluoroquinolones, linking farm use to human infections.
These examples show plasmids bridging environments, from farms to clinics.
Here’s a table of notable real-world cases:
| Pathogen | Plasmid Involved | Resistance/Virulence Traits | Location/Year | Impact | Spread Mechanism |
|---|---|---|---|---|---|
| Shigella dysenteriae | NR1-like R-plasmid | Multi-drug (sulfa, strep, tet) | Japan, 1950s | Dysentery epidemic | Conjugation in communities |
| Escherichia coli | IncFII with mcr-1 | Colistin resistance | Worldwide, 2015+ | Last-resort antibiotic failure | Farm-to-human via food |
| Klebsiella pneumoniae | pOXA-48 | Carbapenem resistance | Europe/Middle East, 2000s | Hospital outbreaks | Nosocomial conjugation |
| Staphylococcus aureus | pSK41 | Methicillin, vancomycin | Global hospitals | MRSA pandemics | Transformation in wounds |
| Pseudomonas aeruginosa | Degradative + resistance hybrid | Hydrocarbons + antibiotics | Oil-polluted sites | Environmental persistence | Biofilm HGT |
| Yersinia pestis | pPCP1 virulence plasmid | Plasminogen activator | Historical plagues | Tissue invasion | Flea-vector transmission |
This highlights the global, interconnected nature of the problem.
Implications of Plasmid-Mediated Resistance in Medicine and the Environment
In medicine, plasmid resistance complicates treatments, leading to longer hospital stays, higher costs, and increased mortality. With fewer effective antibiotics, routine surgeries become risky, echoing pre-antibiotic eras.
Environmentally, plasmids in soil and water create resistance reservoirs, amplified by pollution. Heavy metals co-select for antibiotic resistance, as genes often co-occur on plasmids.
One Health perspective: Resistance flows between animals, humans, and nature, demanding integrated solutions like better stewardship and surveillance.
- Medical Challenges:
- Rise of pan-resistant strains.
- Need for new drugs or phage therapy.
- Environmental Concerns:
- Wastewater as hotspots for HGT.
- Impact on wildlife microbiomes.
Forward-thinking: CRISPR-based tools could target plasmids, or eco-friendly alternatives reduce selection pressure.
In conclusion, R-factors and plasmids are ingenious bacterial tools turned against us through misuse. Understanding them deeply is our best defense, fostering innovations to preserve antibiotics for future generations. This evolving field reminds us that bacteria, though tiny, are masterful survivors.
Read These Articles in Detail
- The Cell: The Fundamental Building Block of Life
- Prokaryotic Cells vs. Eukaryotic Cells: A Detailed Exploration
- Prokaryotic Cells: Definition, Anatomy, Functions, and Their Importance
- Eukaryotic Cells Explained: Definition, Structure, Functions, and Importance
- The Cell Membrane: Structure, Functions, and Evolutionary Significance
- The Cell Wall: Structure, Composition, Functions, and Evolutionary Significance
- Endomembrane System: A Detailed Exploration of the Cellular Machinery of Life
- Mitochondria: A Comprehensive Guide to Their Structure and Functions
- The Cell Envelope: The Protective Shield of Prokaryotic Cells
- Ribosomes: Definition, Structure, and Functions
- Endoplasmic Reticulum: Structure, Types, and Functions Explained
- The Golgi Apparatus: Structure, Functions, and Cellular Importance
- Cytoplasm and Nucleus: Structure, Functions, Differences, and Examples
- What Is Cell Signaling? Structure, Types, and Functions Explained
- Three Main Parts of a Nucleotide: Structure and Functions
- Enzymes: Definition, Structure, Classification, and Functions
- DNA: Structure, Function, Discovery, and Modern Applications
- DNA Replication: Steps, Process, and Genetic Importance
- Properties of DNA: Structure, Physical, and Chemical Characteristics
- DNA Packaging: How DNA Fits into the Nucleus of a Cell
- R-Factors and Plasmids: How Bacteria Develop Antibiotic Resistance
- Chromosomes: Structure, Types, Functions, and Role in Genetics
- Genetic Material: DNA, RNA, and Their Role in Heredity and Evolution
- Deoxyribose and Ribose: Structure and Function in DNA and RNA
- Differences Between Genes and DNA: Definitions, Structures, and Functions
- RNA: Definition, Structure, Types, Functions, and Its Role in Life
Frequently Asked Questions
FAQ 1: What are plasmids and how do they function in bacteria?
Plasmids are small, circular pieces of DNA that exist separately from the main bacterial chromosome, acting like add-on genetic modules that give bacteria extra capabilities. They replicate independently, allowing them to be copied and passed on during cell division or shared between bacteria. In simple terms, think of plasmids as portable toolkits that bacteria can use to adapt quickly to their surroundings, whether that’s surviving antibiotics, breaking down toxins, or even infecting hosts more effectively.
These genetic elements are found in many bacteria, archaea, and some eukaryotes, but they’re most studied in bacteria due to their role in rapid evolution. Plasmids vary in size, from a few thousand to hundreds of thousands of base pairs, and can exist in multiple copies per cell, which amplifies their effects. Their functions go beyond basic survival; they often carry genes that provide advantages in harsh environments, like resistance to heavy metals or the ability to metabolize unusual compounds.
- Replication and Maintenance: Plasmids have their own origin of replication, ensuring they duplicate without relying on the chromosome. They also include partitioning systems to distribute copies evenly to daughter cells during division.
- Gene Expression: Genes on plasmids can be turned on or off based on environmental cues, allowing bacteria to express traits only when needed, conserving energy.
- Host Benefits: In natural settings, plasmids might help bacteria colonize new niches, such as degrading hydrocarbons in oil spills or producing toxins to outcompete rivals.
- Potential Drawbacks: Carrying plasmids can sometimes burden the host with extra metabolic costs, but benefits like antibiotic resistance often outweigh this in selective pressures.
In biotechnology, plasmids are harnessed for gene cloning and protein production, but in nature, their ability to spread traits horizontally makes them key players in bacterial communities. Overall, plasmids drive bacterial adaptability, making them both a marvel of evolution and a challenge in fighting infections.
FAQ 2: How were R-factors first discovered in bacteria?
The discovery of R-factors, or resistance plasmids, traces back to the late 1950s in Japan during a series of dysentery epidemics caused by Shigella bacteria. Researchers noticed that these strains were suddenly resistant to multiple antibiotics, like sulfonamides, streptomycin, and tetracycline, which had previously been effective. This wasn’t just random mutations; the resistance was transferable between bacteria, hinting at a mobile genetic element.
Key figures like Tsutomu Watanabe and colleagues pinpointed these as extrachromosomal factors in 1959, initially observed in Shigella isolates from patients. By the early 1960s, experiments confirmed that resistance could spread via conjugation, a process where bacteria connect and exchange DNA. This breakthrough came amid growing antibiotic use post-World War II, highlighting how human practices accelerated natural bacterial adaptations.
- Historical Context: The epidemics in Japan involved multi-drug resistant Shigella, leading to investigations that revealed non-chromosomal resistance.
- Key Experiments: Scientists transferred resistance from resistant to sensitive strains in lab settings, proving it was plasmid-mediated rather than chromosomal.
- Evolution of Understanding: By the 1970s, R-factors were linked to transposons and integrons, explaining how they acquire new genes.
- Global Spread: Similar discoveries soon followed in other countries, showing R-factors in various pathogens like Escherichia coli and Pseudomonas.
This finding revolutionized microbiology, shifting focus to mobile genetics and sparking ongoing research into antibiotic resistance origins. It also underscored the need for prudent antibiotic use to curb such evolutions.
FAQ 3: What is the structure of a typical resistance plasmid?
A typical resistance plasmid, often called an R-plasmid, is a circular DNA molecule ranging from 80 to 95 kilobases in length, designed for efficiency and mobility. It’s divided into two main segments: the resistance transfer factor (RTF), which handles replication and transfer, and the R-determinant, which carries the actual resistance genes. This modular setup allows for easy gene exchange, making these plasmids highly adaptable.
The RTF resembles parts of fertility plasmids, including genes for conjugation machinery like the tra operon and a finO gene that regulates transfer. Flanking the R-determinant are insertion sequences, such as IS1 elements, which act like molecular scissors, enabling the integration or excision of genes. Overall, this structure supports autonomous replication, stability, and spread.
- Core Components: Includes an origin of replication (oriV) for independent copying and partitioning genes (parA/parB) for even distribution.
- Variable Regions: Resistance cassettes vary, encoding enzymes like beta-lactamases or efflux pumps for drug expulsion.
- Mobile Elements: Transposons and integrons allow gene capture from the environment, enhancing versatility.
- Size and Homology: Often homologous to F-plasmids, facilitating compatibility with bacterial hosts.
In essence, this architecture makes R-plasmids efficient vehicles for resistance, contributing to their persistence in diverse bacterial populations. Understanding it helps in designing targeted therapies to disrupt their function.
FAQ 4: What are the main types of bacterial plasmids?
Bacterial plasmids come in several types, each serving distinct roles that enhance bacterial survival and adaptation. They’re classified based on functions like gene transfer, resistance, or virulence, and many hybrids exist, combining traits for greater impact.
Fertility plasmids, or F-plasmids, enable conjugation by coding for sex pili, turning host cells into donors. Resistance plasmids (R-plasmids) provide defense against antibiotics and metals. Colicin plasmids produce bacteriocins to kill competitors. Degradative plasmids break down complex pollutants. Virulence plasmids boost pathogenicity with toxin or adhesion genes. Some plasmids overlap, like those resisting heavy metals while also carrying catabolic functions.
- Fertility Plasmids: Facilitate DNA sharing; example: F-plasmid in E. coli.
- Resistance Plasmids: Confer multi-drug resistance; example: NR1 in Shigella.
- Colicin Plasmids: Produce toxins for competition; example: ColE1 in coliforms.
- Degradative Plasmids: Metabolize xenobiotics; example: TOL plasmid in Pseudomonas.
- Virulence Plasmids: Enhance infection; example: pPCP1 in Yersinia pestis.
- Heavy Metal Plasmids: Detoxify metals; example: pMOL30 in Alcaligenes.
These types illustrate plasmids’ versatility, from ecological roles to clinical challenges. Researchers continue to discover new variants in diverse environments.
FAQ 5: How does horizontal gene transfer contribute to antibiotic resistance?
Horizontal gene transfer (HGT) is a process where bacteria exchange genetic material directly, bypassing traditional inheritance, and it’s a major driver of antibiotic resistance spread. Through mechanisms like conjugation, transformation, and transduction, resistance genes on plasmids move between bacteria, even across species, accelerating resistance evolution.
Conjugation involves physical contact via pili, often mediated by conjugative plasmids. Transformation picks up free DNA from the environment. Transduction uses bacteriophages as vectors. This sharing creates resistant populations quickly, especially in dense settings like guts or hospitals.
- Conjugation’s Role: Most common for plasmid transfer, promoting multi-drug resistance.
- Environmental Factors: Stressors like antibiotics or pollutants boost HGT rates.
- Impact on Resistance: Allows harmless bacteria to pass genes to pathogens.
- Examples: ARG spread in wastewater or farms via HGT.
HGT turns local resistance into global threats, emphasizing the need for surveillance. Strategies targeting HGT could slow this dissemination.
FAQ 6: What is gene amplification in the context of R-plasmids?
Gene amplification in R-plasmids occurs when bacteria exposed to low antibiotic doses duplicate resistance genes, increasing their expression and survival chances. This happens during replication, where recombination between strands creates multiple copies in tandem arrays, ramping up resistance levels temporarily.
In R-determinants, genes like those for efflux pumps multiply, allowing cells to withstand higher drug concentrations. It’s reversible; without pressure, copies reduce, but it provides a bridge to permanent adaptations.
- Mechanism: Low doses trigger duplication via sister-strand recombination.
- Benefits to Bacteria: Enhances resistance without permanent genome changes.
- Real-World Ties: Common in sub-therapeutic antibiotic use in agriculture.
- Research Insights: Studied in models like E. coli with tet genes.
This process highlights how plasmids enable dynamic responses to threats. It contributes to persistent resistance in clinical and environmental settings.
FAQ 7: Can you give real-world examples of plasmid-mediated antibiotic resistance outbreaks?
Plasmid-mediated resistance has fueled numerous outbreaks, turning treatable infections into crises. In 2011, an E. coli O104:H4 outbreak in Europe involved a virulence plasmid carrying Shiga toxin genes, causing over 4,000 cases of hemolytic uremic syndrome. In hospitals, Klebsiella pneumoniae with IncF plasmids spread carbapenem resistance, leading to untreatable pneumonias worldwide.
The mcr-1 gene on plasmids conferred colistin resistance, first noted in China in 2015, spreading globally via food chains. Shigella epidemics in 1950s Japan marked early examples, with R-plasmids transferring multi-drug resistance.
- NDM-1 Superbug: Plasmid-borne, spread from India, resisting beta-lactams.
- Vibrio cholerae in Haiti: Plasmids aided toxin gene transfer post-earthquake.
- Salmonella in Poultry: R-plasmids link farm antibiotics to human cases.
- Enterobacterales in Hospitals: NDM-5 plasmids caused multi-species outbreaks.
These cases show plasmids bridging environments, amplifying public health risks. Global surveillance is crucial to track such events.
FAQ 8: What strategies are being developed to combat plasmid-driven antibiotic resistance?
Combating plasmid-driven resistance involves innovative approaches like plasmid curing, conjugation inhibitors, and CRISPR-based tools to remove or disrupt resistance genes. Plasmid curing uses compounds to displace plasmids without killing hosts, reducing ARG burden. Conjugation inhibitors block transfer, limiting spread.
CRISPR-Cas9 targets plasmids for cleavage, while predatory gene drives eliminate them. Combination therapies suppress double resistance emergence. Environmental measures reduce selection pressures from pollutants.
- Plasmid Curing: Incompatibility functions or fatty acids like linoleic acid displace plasmids.
- CRISPR Applications: Engineered to cut ARG-carrying plasmids in bacteria.
- Inhibitors: Melatonin prevents conjugative transfer.
- Ecological Strategies: Promote plasmid loss in non-selective conditions.
These tactics aim to restore antibiotic efficacy and curb AMR evolution. Ongoing research focuses on safe, scalable implementations.
FAQ 9: What are the beneficial uses of plasmids in biotechnology?
Plasmids are invaluable in biotechnology, serving as vectors for gene cloning, protein production, and therapeutic development. They’re used to insert genes into host cells for expressing proteins like insulin or enzymes, scaling up production in bacteria or yeast.
In gene therapy, plasmids deliver therapeutic genes to treat diseases. DNA vaccines use them to elicit immune responses. Bioremediation employs degradative plasmids to clean pollutants.
- Gene Cloning: Plasmids like pBR322 amplify DNA fragments for research.
- Protein Expression: Produce recombinant proteins for drugs or diagnostics.
- Gene Therapy: Vectors for delivering antiangiogenic genes in cancer treatment.
- Vaccine Development: Platforms for mRNA precursors or direct immunization.
Their versatility drives advances in medicine and industry. Ethical use ensures benefits outweigh risks.
FAQ 10: What is the environmental impact of resistance plasmids?
Resistance plasmids in environments like soil, water, and wastewater create reservoirs of antibiotic resistance genes, spreading to pathogens and disrupting ecosystems. Pollution from antibiotics or metals co-selects for these plasmids, amplifying ARG persistence.
In urban rivers or farms, they facilitate HGT, affecting microbial communities and wildlife. Microplastics boost plasmid transfer rates, worsening dissemination.
- Reservoir Formation: Plasmids in non-pathogenic bacteria serve as ARG sources.
- Ecological Shifts: Alter bacterial competition and nutrient cycling.
- Human-Environment Link: Contaminated water transfers resistance to clinics.
- Climate Ties: Warming may enhance plasmid stability in polar regions.
Addressing this requires reducing pollution and monitoring resistomes. One Health approaches integrate environmental strategies with health policies.
FAQ 11: How Do Plasmids Contribute to Bacterial Evolution and Adaptation?
Plasmids play a pivotal role in shaping bacterial evolution by acting as vehicles for rapid genetic change, allowing bacteria to adapt to new environments far quicker than through chromosomal mutations alone. These small, circular DNA molecules carry accessory genes that aren’t essential for basic survival but provide advantages in specific conditions, such as resisting antibiotics, degrading pollutants, or enhancing virulence. By facilitating horizontal gene transfer, plasmids enable bacteria to share these beneficial traits across populations, even between different species, which accelerates evolutionary processes and promotes diversity within microbial communities.
Beyond just transferring genes, plasmids influence bacterial ecology by introducing variability that can lead to novel phenotypes. For instance, in fluctuating environments, plasmids can carry genes that help bacteria switch between lifestyles, like from free-living to symbiotic states. This modularity means bacteria can acquire, test, and discard traits without risking their core genome, making evolution more experimental and efficient. Recent studies highlight how plasmids not only mobilize genes but also affect host fitness through interactions that can stabilize or destabilize populations, underscoring their broader impact on microbial dynamics.
In the long term, plasmids drive large-scale evolutionary patterns, such as the emergence of multi-resistant strains in clinical settings. Their ability to replicate independently and maintain multiple copies per cell amplifies gene expression, providing a buffer against environmental pressures. This copy number variation can lead to rapid adaptation, where bacteria with higher plasmid loads survive better under stress. Moreover, plasmids can integrate into chromosomes or evolve into stable elements, blurring the lines between mobile and fixed genetics. As bacteria face global challenges like climate change or pollution, plasmids will likely continue to be key players in their resilience and diversification.
Overall, the evolutionary contributions of plasmids extend to ecosystem-level effects, influencing everything from soil nutrient cycling to human health. By promoting gene flow, they ensure bacterial communities remain adaptable, but this also poses risks, such as the swift spread of resistance in pathogens. Understanding these mechanisms is crucial for predicting microbial responses to human interventions and developing strategies to manage them.
FAQ 12: What Role Do Integrons Play in Antibiotic Resistance Plasmids?
Integrons are specialized genetic platforms embedded in plasmids that capture and express antibiotic resistance genes, making them powerful tools for bacteria to build multi-drug resistance arsenals. These structures consist of an integrase gene, a promoter, and attC sites where gene cassettes insert, allowing bacteria to collect resistance traits like puzzle pieces.
- Gene Capture Mechanism: Integrons use site-specific recombination to incorporate exogenous gene cassettes, often from the environment, enabling quick adaptation to new antibiotics without needing mutations.
- Expression Control: A strong promoter in the integron ensures captured genes are expressed efficiently, turning silent DNA into active resistance factors.
- Association with Mobile Elements: Frequently found on plasmids or transposons, integrons facilitate horizontal transfer, spreading resistance across bacterial species.
- Class 1 Integrons Dominance: The most common type in clinical settings, class 1 integrons often carry multiple cassettes for resistances like beta-lactams or aminoglycosides, contributing to superbugs.
- Evolutionary Impact: By promoting gene shuffling, integrons accelerate resistance evolution, with studies showing their prevalence in polluted sites where selection pressure is high.
- Detection and Monitoring: Tools like PCR target integron sequences to track resistance dissemination, aiding surveillance in hospitals and farms.
- Therapeutic Targets: Inhibiting integrase activity could disrupt integron function, offering a way to curb resistance buildup in plasmids.
These features make integrons central to the resistance crisis, as they allow plasmids to evolve dynamically.
FAQ 13: What Are the Differences Between Conjugative, Mobilizable, and Non-Conjugative Plasmids?
Conjugative, mobilizable, and non-conjugative plasmids differ mainly in their ability to transfer between bacteria, impacting how traits like resistance spread. The table below compares their key characteristics, sizes, mechanisms, and examples for a clear overview.
| Plasmid Type | Transfer Ability | Key Features | Typical Size Range | Transfer Mechanism | Common Examples | Role in Resistance Spread |
|---|---|---|---|---|---|---|
| Conjugative | Self-transferable; can initiate and complete transfer independently | Encodes full conjugation machinery, including tra genes for pilus and relaxase | 50-200 kb or larger | Direct cell-to-cell conjugation via sex pilus | F-plasmid, IncP-1 plasmids like RK2 | High; drives widespread dissemination of resistance genes across species |
| Mobilizable | Transferable only with help from conjugative plasmids | Encodes relaxase and oriT site but lacks full tra operon | 5-50 kb, generally smaller | Hitchhikes on conjugative plasmids’ machinery during co-transfer | ColE1, pRSF1010 | Moderate; depends on co-resident conjugative plasmids for mobility |
| Non-Conjugative | Cannot transfer independently or with help | Lacks both tra genes and mobilization elements like oriT | Variable, often 1-100 kb | None; relies on transformation, transduction, or integration | pUC series (lab vectors), some cryptic plasmids | Low; stable in host but doesn’t spread horizontally easily |
This classification highlights how conjugative plasmids are the most mobile, posing greater risks for resistance epidemics, while non-conjugative ones are more host-specific.
FAQ 14: How Are Bacterial Plasmids Isolated and Analyzed in Laboratories?
Isolating and analyzing plasmids in labs involves a series of steps to extract, purify, and characterize these DNA elements, essential for studying resistance or genetic engineering. Common methods combine biochemistry with molecular tools for precise results.
- Bacterial Culture Growth: Start by growing bacteria in selective media overnight to amplify plasmid copy numbers, ensuring enough material for extraction.
- Cell Harvesting and Lysis: Centrifuge to pellet cells, then lyse using alkaline solutions or enzymes to release plasmids while denaturing chromosomal DNA.
- Purification Techniques: Use silica-based columns or cesium chloride gradients to separate plasmids from contaminants like RNA or proteins.
- Quantification and Verification: Measure DNA concentration with spectrophotometry, then confirm plasmid presence via agarose gel electrophoresis.
- Restriction Digest Analysis: Cut plasmids with enzymes to generate fragment patterns, verifying size and structure against expected maps.
- Sequencing and PCR: Employ next-generation sequencing for full genomes or PCR to detect specific genes, like resistance markers.
- Advanced Tools: Techniques such as pulsed-field gel electrophoresis separate large plasmids, while qPCR quantifies copy numbers in cells.
- Bioinformatics Integration: Analyze sequences with software to identify origins, genes, and mobility elements for comprehensive profiling.
These protocols enable researchers to track plasmid evolution and develop countermeasures.
FAQ 15: What Is the Impact of Resistance Plasmids on the Human Gut Microbiome?
The human gut microbiome, a complex ecosystem of trillions of microbes, faces significant disruption from resistance plasmids, which alter community dynamics and health outcomes. These plasmids introduce antibiotic resistance genes that can spread among commensal bacteria, turning the gut into a reservoir for resistant pathogens. This not only complicates treatments for infections but also affects microbial balance, potentially leading to dysbiosis where harmful bacteria outcompete beneficial ones.
In everyday scenarios, exposure to antibiotics through food or medicine selects for plasmid-carrying bacteria, allowing them to persist and transfer genes horizontally. This process can reduce microbiome diversity, impairing functions like nutrient absorption or immune modulation. For example, in individuals with frequent antibiotic use, resistance plasmids may amplify, fostering environments where opportunistic pathogens thrive, increasing risks of conditions like Clostridium difficile infections.
Long-term, the presence of these plasmids influences host physiology, as resistant microbes might produce altered metabolites affecting inflammation or metabolism. Studies show that global travel and diet variations exacerbate plasmid dissemination, linking urban lifestyles to higher resistance loads in gut flora. Moreover, plasmids can carry non-resistance genes that enhance virulence or colonization, further entrenching imbalances.
Addressing this impact requires holistic approaches, like probiotic interventions to restore diversity or targeted therapies to eliminate plasmids without harming the microbiome. As resistance grows, understanding these effects is vital for preserving gut health and preventing broader public health crises.
FAQ 16: What Are the Emerging Threats from Plasmid-Mediated Antibiotic Resistance in 2025?
As of 2025, plasmid-mediated resistance continues to evolve, presenting new challenges with genes like fosA4 spreading in wildlife and clinical settings, amplifying threats to last-resort antibiotics.
- Novel Gene Combinations: Plasmids increasingly carry hybrid resistance cassettes, such as those for fosfomycin and colistin, complicating treatments for Gram-negative infections.
- Environmental Reservoirs: Pollution boosts plasmid persistence in water and soil, facilitating transfer to human pathogens via food chains.
- Insertion Sequence Activity: Elements like IS1 in plasmids accelerate resistance acquisition by promoting gene insertions, as seen in pOXA-48 variants.
- Global Economic Strain: Rising healthcare costs from resistant infections, projected at billions annually, underscore the need for urgent interventions.
- Vaccine and Potentiator Gaps: While new strategies emerge, plasmids evade them through rapid adaptation, demanding innovative anti-plasmid approaches.
- One Health Implications: Cross-species transmission, especially in agriculture, heightens risks, with broad-host-range plasmids linking animal and human resistomes.
- Surveillance Challenges: Undetected silent carriers in communities allow stealthy spread, calling for advanced genomic monitoring.
These threats highlight the dynamic nature of plasmid evolution, urging multidisciplinary responses.
FAQ 17: Do Plasmids Exist in Archaea and Eukaryotes, and How Do They Compare to Bacterial Ones?
Plasmids are not exclusive to bacteria; they occur in archaea and some eukaryotes, though with differences in structure, function, and prevalence. The table below outlines key comparisons across domains.
| Domain | Plasmid Presence | Typical Characteristics | Size and Shape | Functions | Examples | Evolutionary Notes |
|---|---|---|---|---|---|---|
| Bacteria | Abundant in many species | Circular, double-stranded, often conjugative | 1-200 kb+ | Resistance, virulence, metabolism | F-plasmid, R-plasmids | Drive horizontal transfer, key in adaptation |
| Archaea | Common, especially in extremophiles | Circular or linear, diverse replication strategies | 5-100 kb | Adaptation to harsh environments, gene transfer | pNRC100 in Halobacterium | Similar to bacterial but with unique proteins |
| Eukaryotes | Rare, mostly in yeast or organelles | Linear or circular, less mobile | 2-100 kb | Maintenance in fungi, mitochondrial DNA-like | 2-micron plasmid in Saccharomyces | Less diverse, often stable without conjugation |
This shows plasmids’ universal role in genetics, with bacterial ones being the most mobile and impactful.
FAQ 18: How Do Plasmids Influence Bacterial Biofilm Formation?
Bacterial biofilms, those slimy communities on surfaces, often get a boost from plasmids that carry genes enhancing adhesion, matrix production, or communication. Plasmids can encode pili or exopolysaccharides, helping bacteria stick together and form protective layers against antibiotics and immune attacks. This interplay makes biofilms hotspots for gene exchange, where plasmids thrive and evolve.
In many pathogens, conjugative plasmids promote biofilm initiation by providing adhesion factors, turning transient clusters into robust structures. For example, in Pseudomonas, plasmids might upregulate quorum-sensing genes, coordinating group behaviors that strengthen the biofilm. This not only shields bacteria but also facilitates plasmid transfer within the dense community, accelerating resistance spread.
Over time, plasmids can modulate biofilm maturity, with some inducing dispersal to colonize new sites. However, carrying these plasmids might impose costs, like reduced motility, balanced by survival benefits in hostile environments. Research shows that in clinical infections, plasmid-laden biofilms complicate treatments, prolonging illnesses.
Ultimately, targeting plasmid-biofilm links could yield new therapies, disrupting these resilient formations at their genetic roots.
FAQ 19: What Is the Economic Impact of Plasmid-Driven Antibiotic Resistance?
Plasmid-mediated resistance imposes hefty economic burdens through healthcare costs, productivity losses, and innovation demands. The table details key impacts, drawing from global estimates.
| Impact Category | Description | Estimated Annual Cost (USD) | Affected Sectors | Contributing Factors | Mitigation Potential |
|---|---|---|---|---|---|
| Healthcare Expenses | Increased hospital stays, advanced treatments for resistant infections | 20-55 billion globally | Hospitals, clinics | Plasmid spread in nosocomial settings | High with surveillance |
| Productivity Losses | Sick days, mortality reducing workforce | Up to 100 billion by 2050 | Economy-wide | Chronic infections from resistant strains | Moderate via prevention |
| Drug Development | R&D for new antibiotics amid resistance | 1-5 billion per drug | Pharma industry | Plasmids accelerating obsolescence | High with incentives |
| Agriculture and Food | Livestock treatments, crop losses | 10-20 billion | Farming, supply chains | Plasmid transfer in animals | Significant with alternatives |
| Poverty Amplification | Disproportionate effects in low-income areas | Variable, trillions long-term | Developing nations | Global plasmid dissemination | Crucial through equity programs |
These figures emphasize the urgent need for investment in curbing plasmid spread to avert escalating costs.
FAQ 20: What Strategies Can Prevent the Spread of Antibiotic Resistance Plasmids?
Preventing plasmid spread requires multifaceted approaches focusing on hygiene, stewardship, and innovation to disrupt transfer and persistence.
- Antibiotic Stewardship Programs: Limit unnecessary prescriptions in medicine and agriculture to reduce selection pressure favoring plasmid-carrying bacteria.
- Plasmid Curing Agents: Use compounds like acridine dyes or fatty acids to displace plasmids from hosts without killing beneficial microbes.
- Conjugation Inhibitors: Develop drugs targeting tra genes or pili to block horizontal transfer during conjugation.
- Hygiene and Infection Control: Promote handwashing, sanitation, and isolation protocols in hospitals to minimize bacterial contact and plasmid exchange.
- Environmental Management: Treat wastewater and reduce metal pollution, as co-selection maintains resistance plasmids in ecosystems.
- Surveillance Systems: Implement genomic monitoring to track plasmid movements and predict outbreaks early.
- Alternative Therapies: Explore phages or CRISPR to specifically eliminate resistance plasmids from populations.
- Public Education: Raise awareness on proper antibiotic use to curb community-level spread.
These strategies, when combined, can slow the resistance tide effectively.
Acknowledgement
The Examsmeta website would like to expresses its gratitude to the various reputable sources that provided invaluable information for the article “R-Factors and Plasmids: How Bacteria Develop Antibiotic Resistance.” Their comprehensive and well-researched content helped shape a detailed and accurate exploration of plasmids and their role in antibiotic resistance. Specifically, acknowledges the contributions of the following platforms for their reliable scientific insights, which enriched the depth and clarity of this work.
- Nature (www.nature.com): For offering peer-reviewed articles and reviews on plasmid biology and antibiotic resistance mechanisms, providing a strong foundation for understanding genetic structures and their implications.
- ScienceDirect (www.sciencedirect.com): For its extensive database of research papers detailing plasmid transfer mechanisms and their environmental impacts, crucial for the article’s depth.
- PubMed (pubmed.ncbi.nlm.nih.gov): For access to a vast array of studies on bacterial evolution and resistance plasmid functions, enabling precise and evidence-based content.
- ASM Journals (journals.asm.org): For specialized microbiology research that illuminated the historical and structural aspects of R-factors, enhancing the article’s scientific rigor.

