Ribonucleic acid, commonly known as RNA, is a vital molecule in all living organisms. It plays a crucial role in protein synthesis, gene regulation, and even acts as a catalyst in some reactions. Unlike its more famous counterpart, DNA, RNA is typically single-stranded and more versatile, allowing it to perform a wide array of functions within cells. From carrying genetic instructions to building proteins, RNA is essential for life as we know it.
This article dives deep into what RNA is, how it works, its various types, and its significance in biology, drawing from established scientific understanding to provide a thorough overview.
Table of Contents
In simple terms, RNA helps translate the genetic code stored in DNA into functional proteins that carry out most cellular activities. It is found in every cell, from bacteria to humans, and even in some viruses where it serves as the genetic material itself. Scientists have been studying RNA for over a century, uncovering its complexities and applications, including in modern medicine like vaccines. Whether you’re a student, a curious reader, or someone interested in biotechnology, understanding RNA opens a window into the molecular machinery of life.
The Central Dogma: RNA’s Core Role in Molecular Biology
At the heart of molecular biology lies the central dogma, a principle that describes the flow of genetic information in cells. According to this concept, DNA holds the genetic blueprint, which is transcribed into RNA, and then RNA is translated into proteins. This process ensures that genetic instructions are accurately passed on and executed to maintain life.
DNA and RNA together form the nucleic acids, one of the major classes of macromolecules essential for all organisms, alongside proteins, lipids, and sometimes carbohydrates. These macromolecules are large molecules built from repeating subunits. For nucleic acids, those subunits are nucleotides, each consisting of a sugar, a phosphate group, and a base.
The central dogma highlights RNA’s teamwork with DNA. DNA’s information is transcribed into RNA, which then carries that message to the protein-making factories in the cell. This flow is unidirectional in most cases, though some exceptions exist, like in certain viruses where RNA can be reverse-transcribed back into DNA.
Proteins, the end products, perform countless tasks, from building tissues to fighting infections. Without RNA bridging DNA and proteins, this vital process would halt, underscoring RNA’s indispensable position in biology.
To illustrate, consider how a cell responds to a signal, like insulin in the bloodstream. The DNA gene for insulin response is transcribed into mRNA, which directs the synthesis of proteins that help cells absorb glucose. This seamless information transfer exemplifies the central dogma in action.
History of RNA: From Discovery to Modern Insights
The story of RNA begins in the late 19th century. In 1868, Swiss scientist Friedrich Miescher isolated a substance from cell nuclei, which he called “nuclein.” This material turned out to be nucleic acids, including both DNA and RNA. At the time, its importance was unclear, but it laid the foundation for future research.
By the early 20th century, scientists realized that nucleic acids were present not just in eukaryotic cells with nuclei but also in prokaryotes like bacteria, which lack a defined nucleus. In 1939, researchers suspected RNA’s involvement in protein synthesis, a hunch that proved correct.
The 1950s and 1960s were pivotal, with the discovery of messenger RNA (mRNA) by Jacob and Monod, and the elucidation of the genetic code. The central dogma was formalized by Francis Crick in 1958, cementing RNA’s role.
A major breakthrough came in the 1980s when Sidney Altman and Thomas Cech discovered that RNA could act as an enzyme, earning them the Nobel Prize in Chemistry in 1989. This finding expanded RNA’s perceived capabilities beyond mere information carrier.
In recent decades, RNA research has exploded. The RNA world hypothesis, proposed in the 1980s, suggests that RNA was the first molecule of life, capable of both storing information and catalyzing reactions. Advances in sequencing technologies have revealed thousands of non-coding RNAs that regulate genes.
The 21st century brought RNA into the spotlight with mRNA vaccines, like those for COVID-19, developed rapidly in 2020. These vaccines use synthetic mRNA to instruct cells to produce viral proteins, triggering immunity. As of 2025, ongoing research explores RNA’s potential in treating genetic diseases and cancer.
Historical milestones include the mapping of the human genome in 2003, which highlighted RNA’s regulatory roles, and the discovery of CRISPR-Cas systems, some of which target RNA.
| Historical Milestone | Year | Key Discovery or Event | Significance |
|---|---|---|---|
| Isolation of Nuclein | 1868 | Friedrich Miescher extracts nucleic acids from white blood cells | First identification of what would become known as DNA and RNA |
| RNA in Protein Synthesis Suspected | 1939 | Early experiments link RNA to protein production | Sets stage for understanding RNA’s functional role |
| Messenger RNA Identified | 1961 | Jacob and Monod describe mRNA | Explains how genetic info moves from DNA to ribosomes |
| Central Dogma Formalized | 1958 | Francis Crick proposes the flow: DNA to RNA to protein | Provides a framework for molecular biology |
| RNA as Enzyme (Ribozymes) | 1980s | Altman and Cech’s work on catalytic RNA | Nobel Prize; shows RNA can function like proteins |
| RNA World Hypothesis | 1986 | Walter Gilbert coins the term | Suggests RNA predated DNA in early life |
| mRNA Vaccines | 2020 | First widespread use against COVID-19 | Demonstrates RNA’s therapeutic potential |
| CRISPR RNA Editing | 2010s | Development of RNA-targeting CRISPR tools | Enables precise gene regulation at RNA level |
This table captures the evolution of RNA knowledge, showing how each discovery built on the last.
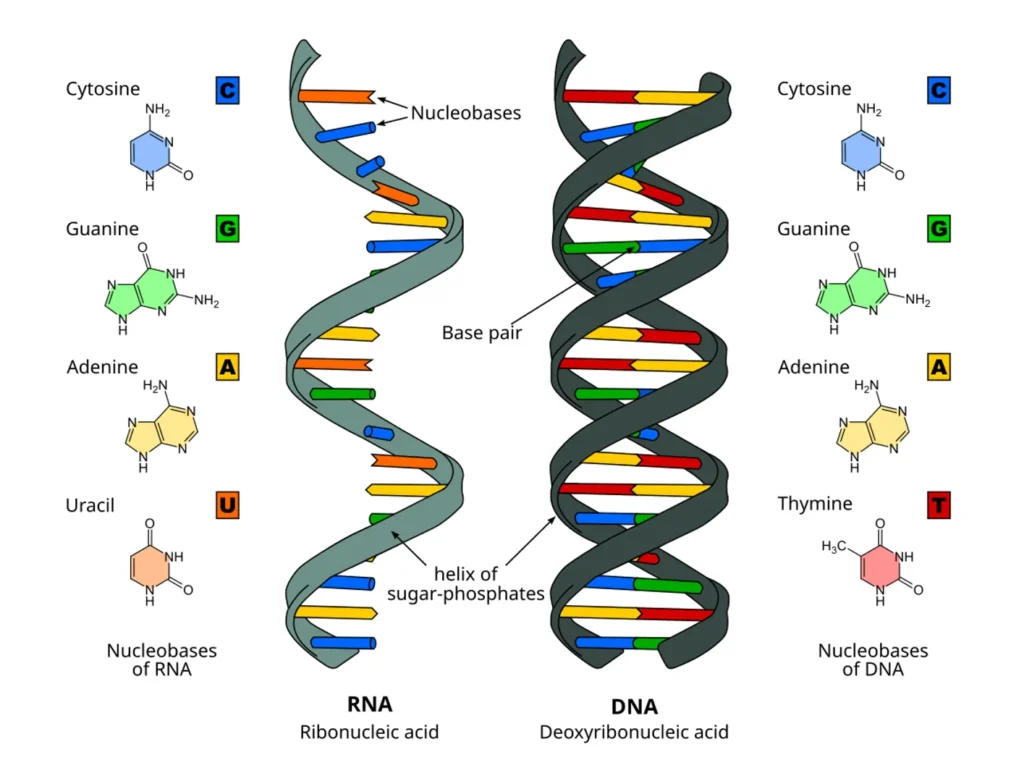
Structure of RNA: Building Blocks and Unique Features
RNA’s structure is similar to DNA but with key differences that enable its diverse functions. Each RNA molecule is a polymer of nucleotides, linked by phosphodiester bonds. A nucleotide has three parts: a ribose sugar, a phosphate group, and a nitrogenous base.
The ribose sugar in RNA has an extra oxygen atom compared to deoxyribose in DNA, making RNA less stable but more flexible. This instability allows RNA to fold into complex shapes, essential for its roles.
The bases in RNA are adenine (A), guanine (G), cytosine (C), and uracil (U), unlike DNA’s thymine (T). Bases pair complementarily: A with U, and G with C, via hydrogen bonds. This pairing enables RNA to form double-stranded regions or hybridize with DNA.
Most RNA is single-stranded, folding into hairpin loops, bulges, and other secondary structures. These can further assemble into tertiary structures, like in tRNA’s cloverleaf shape.
For example, ribosomal RNA (rRNA) forms large complexes with proteins in ribosomes, while transfer RNA (tRNA) has a distinctive L-shape for amino acid binding.
RNA can also form double-stranded molecules, as in some viruses or regulatory RNAs like siRNA.
The length of RNA varies: mRNA can be thousands of nucleotides long, while small regulatory RNAs are just dozens.
| Component | Description | Role in Structure |
|---|---|---|
| Ribose Sugar | Five-carbon sugar with a hydroxyl group at the 2′ position | Provides the backbone; extra oxygen increases reactivity and flexibility |
| Phosphate Group | Links sugars via phosphodiester bonds | Forms the chain; negatively charged, affecting solubility |
| Nitrogenous Bases | Adenine (A), Guanine (G), Cytosine (C), Uracil (U) | Carry genetic information; enable base pairing and folding |
| Secondary Structures | Hairpins, loops, stems | Allow functional shapes, like catalytic sites in ribozymes |
| Tertiary Structures | 3D folding, often stabilized by ions | Enable interactions with proteins and other molecules |
This table breaks down RNA’s structural elements, highlighting their contributions.
Key Differences Between RNA and DNA
While RNA and DNA are both nucleic acids, they differ in ways that suit their roles. DNA is double-stranded and stable, ideal for long-term genetic storage, whereas RNA is single-stranded and transient, perfect for dynamic processes.
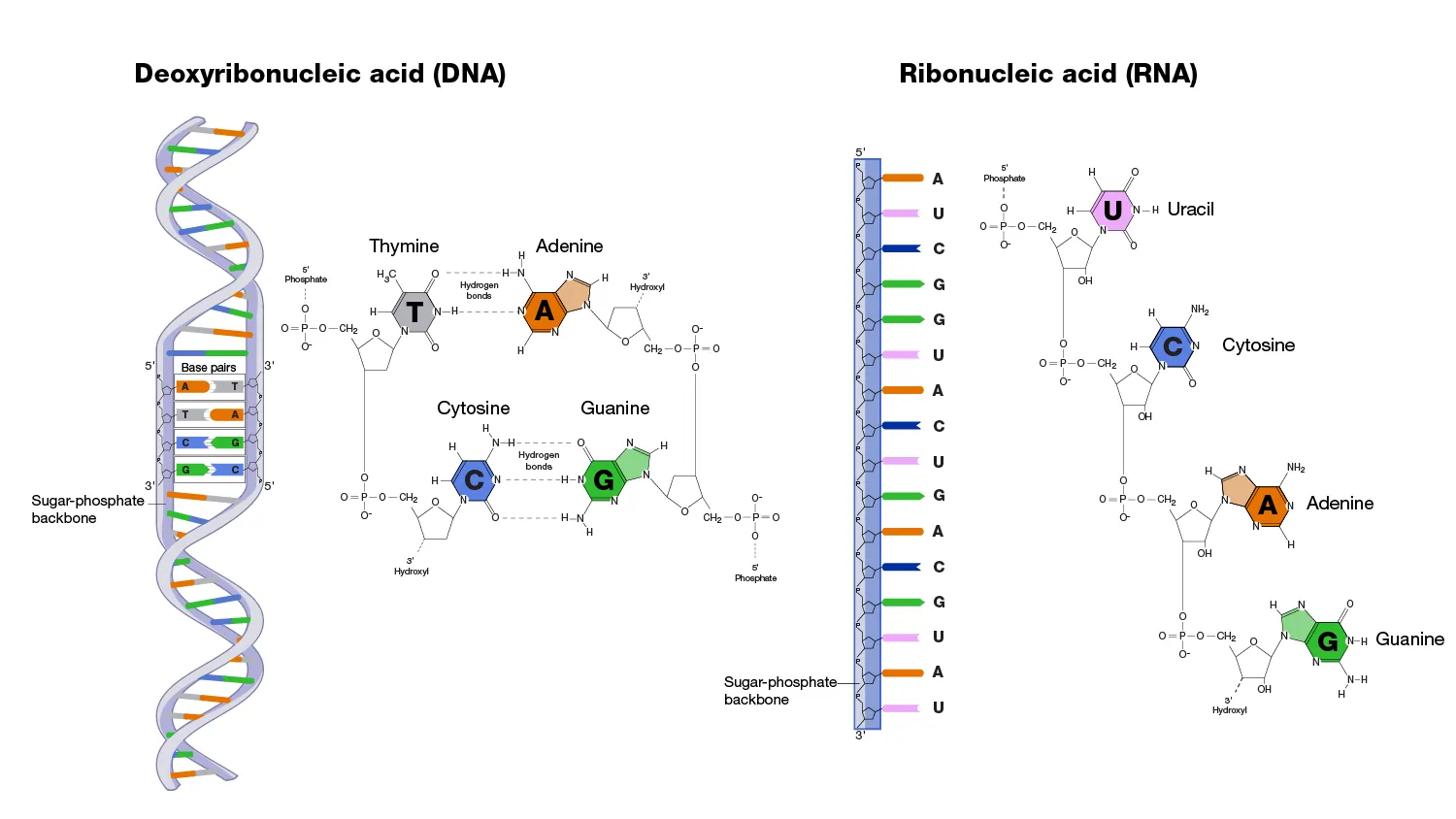
DNA uses deoxyribose sugar, lacking RNA’s 2′ hydroxyl group, making it more resistant to hydrolysis. RNA’s bases include uracil instead of thymine, which pairs similarly but is easier to synthesize.
Location-wise, DNA resides in the nucleus (in eukaryotes), while RNA is produced there but functions in the cytoplasm. DNA is typically much longer, forming chromosomes, whereas RNA molecules are shorter and more varied.
Functionally, DNA stores information, but RNA transcribes, translates, and regulates it. These differences evolved to optimize cellular efficiency.
For instance, in viruses, some use RNA genomes because it’s quicker to replicate, allowing faster mutation and adaptation.
| Feature | RNA | DNA |
|---|---|---|
| Strands | Usually single-stranded | Double-stranded helix |
| Sugar | Ribose | Deoxyribose |
| Bases | A, G, C, U | A, G, C, T |
| Stability | Less stable, prone to degradation | Highly stable |
| Length | Varies, often short (e.g., 70-10,000 nucleotides) | Very long (millions to billions) |
| Location | Cytoplasm, nucleus, organelles | Primarily nucleus |
| Primary Function | Protein synthesis, regulation, catalysis | Genetic storage |
| Examples | mRNA, tRNA, rRNA | Chromosomal DNA, mitochondrial DNA |
This comparison table illustrates the contrasts, aiding in understanding their complementary roles.
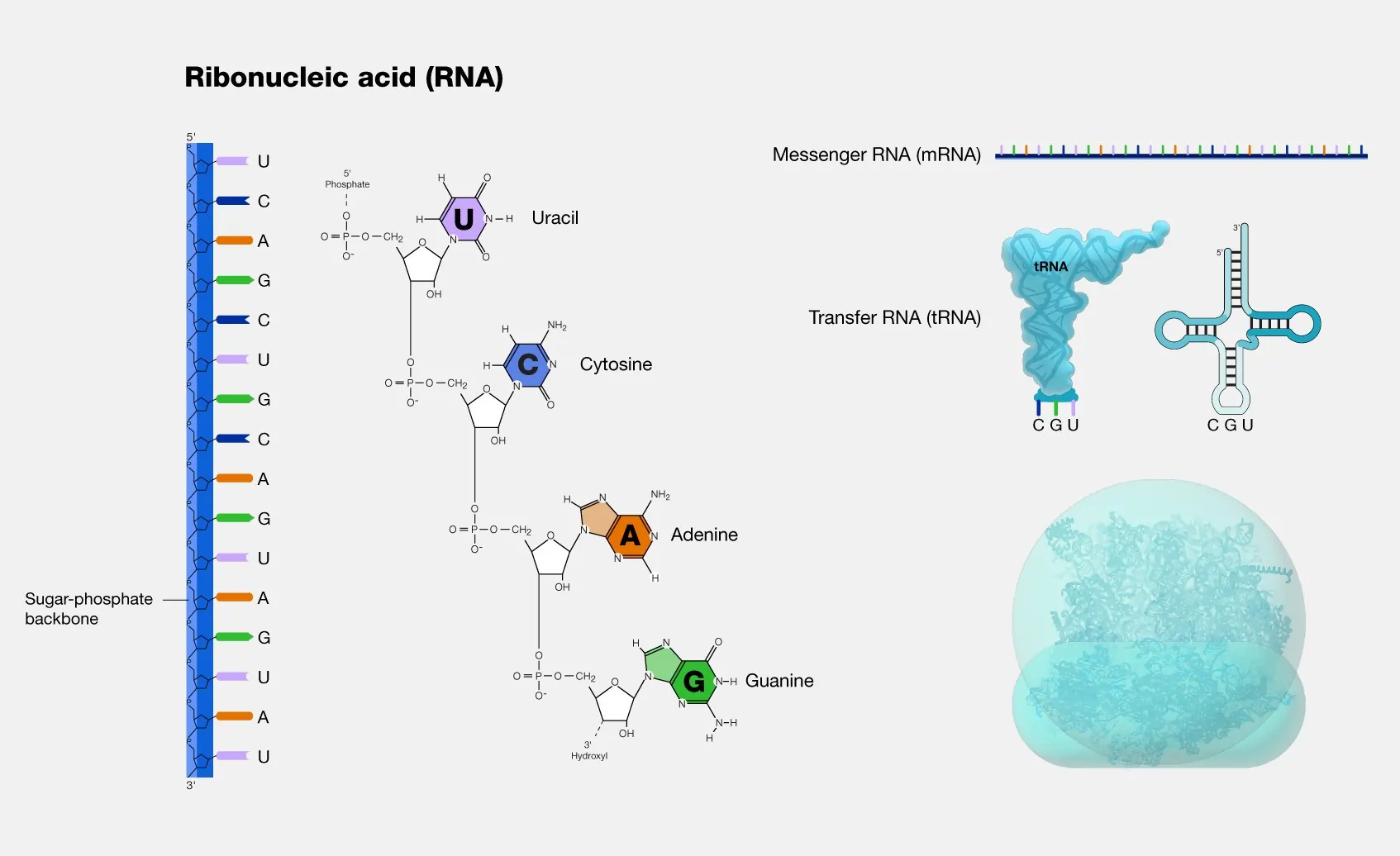
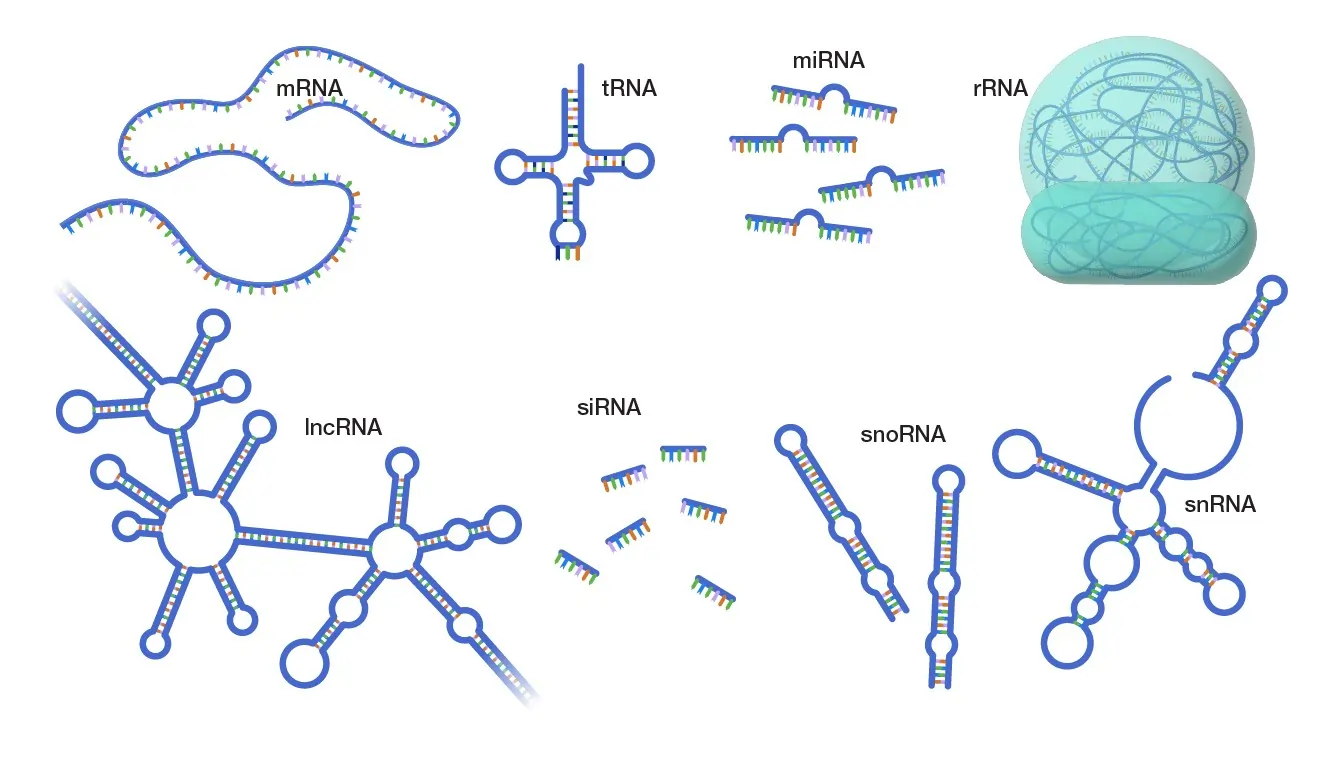
Types of RNA: Diversity in Form and Function
RNA comes in many types, each specialized for specific tasks. The three main ones involved in protein synthesis are messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA). Beyond these, there are regulatory and catalytic RNAs.
Messenger RNA (mRNA) carries the genetic code from DNA to ribosomes. It’s transcribed from genes and includes codons that specify amino acids. In eukaryotes, mRNA is processed by adding a cap and tail, and splicing out introns.
Transfer RNA (tRNA) acts as an adapter, linking amino acids to their codons on mRNA. Each tRNA has an anticodon that matches the mRNA codon and binds a specific amino acid.
Ribosomal RNA (rRNA) forms the core of ribosomes, the protein assembly sites. It catalyzes peptide bond formation, acting as a ribozyme.
Other types include:
- Small nuclear RNA (snRNA): Involved in splicing mRNA.
- MicroRNA (miRNA): Short RNAs that regulate gene expression by binding to mRNA and preventing translation.
- Long non-coding RNA (lncRNA): Regulate chromatin structure and gene activity.
- Small interfering RNA (siRNA): Trigger RNA interference to silence genes.
- Piwi-interacting RNA (piRNA): Protect germ cells from transposons.
These types showcase RNA’s versatility.
For example, miRNAs are crucial in development; dysregulation can lead to diseases like cancer.
| Type of RNA | Abbreviation | Function | Location | Example Role |
|---|---|---|---|---|
| Messenger RNA | mRNA | Carries genetic info from DNA to ribosomes | Nucleus to cytoplasm | Codes for insulin protein |
| Transfer RNA | tRNA | Brings amino acids to ribosomes | Cytoplasm | Matches codons during translation |
| Ribosomal RNA | rRNA | Structural and catalytic component of ribosomes | Ribosomes in cytoplasm | Forms peptide bonds |
| Small Nuclear RNA | snRNA | mRNA splicing | Nucleus | Removes introns from pre-mRNA |
| MicroRNA | miRNA | Gene regulation by mRNA degradation or translation inhibition | Cytoplasm | Suppresses oncogenes in cancer prevention |
| Long Non-Coding RNA | lncRNA | Regulates gene expression, chromatin modification | Nucleus and cytoplasm | X-chromosome inactivation |
| Small Interfering RNA | siRNA | RNA interference, gene silencing | Cytoplasm | Antiviral defense in plants |
| Piwi-Interacting RNA | piRNA | Transposon silencing in germline | Germ cells | Maintains genome integrity |
This extensive table lists major RNA types, their abbreviations, functions, locations, and real-world examples.
Functions of RNA: From Information Carrier to Regulator
RNA’s functions extend far beyond protein synthesis. It acts as a messenger, adapter, catalyst, and regulator, influencing nearly every cellular process.
Primary functions include:
- Facilitating DNA translation into proteins by providing the template.
- Serving as an adapter in protein synthesis via tRNA.
- Acting as a messenger between DNA and ribosomes through mRNA.
- Carrying genetic information in some organisms, like RNA viruses.
- Helping ribosomes select correct amino acids for protein building.
Additionally, RNA regulates gene expression. Non-coding RNAs fine-tune when and how genes are active, crucial for development and response to environments.
In catalysis, ribozymes like rRNA in ribosomes speed up reactions without proteins. This enzymatic role supports the idea that RNA was central in early life.
RNA also plays in immunity: siRNA and miRNA defend against viruses by degrading foreign RNA.
In biotechnology, synthetic RNA is used in therapies. For instance, mRNA vaccines introduce harmless viral mRNA, prompting cells to produce antigens for immune training.
Consider HIV, an RNA virus: Its genome is reverse-transcribed into DNA, integrating into host cells, showing RNA’s adaptability.
RNA functions in energy metabolism too, like in riboswitches that sense metabolites and alter gene expression.
Overall, RNA’s multifaceted roles make it a cornerstone of biology.
RNA as an Enzyme: The Discovery of Ribozymes
Traditionally, enzymes were thought to be proteins, but the 1980s revelation that RNA can catalyze reactions changed that. Ribozymes, RNA enzymes, perform tasks like cleaving bonds or joining molecules.
The peptidyl transferase center in ribosomes, made of rRNA, catalyzes protein synthesis, perhaps the most critical ribozyme.
Other examples: snRNAs in spliceosomes remove introns from mRNA, and self-splicing introns in some genes.
RNA’s flexibility, due to its ribose sugar, allows shape-shifting for catalysis, unlike stable DNA.
This discovery bolstered the RNA world hypothesis, suggesting life began with self-replicating RNA.
In medicine, ribozymes are engineered to target viral RNAs, offering potential treatments for diseases like hepatitis.
RNA as a Regulator: Controlling Gene Expression
Beyond coding, much RNA is non-coding, regulating genes at multiple levels.
miRNAs bind to mRNA, blocking translation or causing degradation, fine-tuning protein levels.
lncRNAs scaffold chromatin modifiers, influencing which genes are accessible.
Riboswitches in bacterial mRNA sense small molecules and change structure to control transcription or translation.
These mechanisms ensure precise gene control, vital for differentiation, where stem cells become specialized.
Dysregulation leads to disorders: Overactive miRNAs can promote cancer by silencing tumor suppressors.
In plants, RNA regulation helps respond to stresses like drought.
RNA Genomes: In Viruses and Viroids
Some organisms use RNA as genetic material. RNA viruses, like influenza or SARS-CoV-2, have RNA genomes replicated by viral enzymes.
Positive-sense RNA viruses use their genome directly as mRNA, while negative-sense need transcription first.
Viroids, tiny circular RNAs, infect plants without coding proteins; they hijack host machinery for replication.
These highlight RNA’s informational capacity, similar to DNA but more mutable.
Double-stranded RNA (dsRNA) in some viruses triggers immune responses in hosts, like interferon production.
In eukaryotes, dsRNA activates RNA interference, silencing genes.
The RNA World Hypothesis: Origins of Life
The RNA world hypothesis posits that RNA was the first self-replicating molecule, predating DNA and proteins. RNA can store info and catalyze, fulfilling both roles.
Evidence: Ribozymes exist today, and prebiotic chemistry could produce RNA nucleotides.
This era might have seen RNA evolving into DNA for stability and proteins for efficiency.
Lab experiments create self-replicating ribozymes, supporting the idea.
It explains life’s transition from simple molecules to complex cells.
RNA in Biotechnology: mRNA Vaccines and Therapies
RNA’s applications in biotech are transformative. mRNA vaccines, pioneered for COVID-19, deliver synthetic mRNA encoding antigens, safe as they don’t integrate into DNA.
They train immunity without live virus, enabling quick development for emerging diseases.
RNA interference therapies, like patisiran for amyloidosis, use siRNA to silence faulty genes.
CRISPR-Cas13 targets RNA, editing transcripts without altering DNA.
Future uses include cancer vaccines and personalized medicine.
As of 2025, RNA drugs treat rare diseases, with trials for common ones like heart conditions.
Recent Discoveries in RNA Research
Advancements continue. In 2023, researchers mapped RNA modifications, or epitranscriptome, affecting stability and function.
Circular RNAs, once junk, now known as regulators, sponging miRNAs.
AI predicts RNA structures, accelerating drug design.
In 2024, new ribozymes were engineered for synthetic biology.
These findings promise deeper insights into diseases and novel treatments.
Conclusion
RNA is more than a intermediary; it’s a dynamic molecule shaping life. From ancient origins to cutting-edge therapies, its study reveals biology’s intricacies. As research progresses, RNA will unlock more secrets, enhancing health and understanding evolution. Exploring RNA reminds us of life’s molecular elegance.
Read These Articles in Detail
- The Cell: The Fundamental Building Block of Life
- Prokaryotic Cells vs. Eukaryotic Cells: A Detailed Exploration
- Prokaryotic Cells: Definition, Anatomy, Functions, and Their Importance
- Eukaryotic Cells Explained: Definition, Structure, Functions, and Importance
- The Cell Membrane: Structure, Functions, and Evolutionary Significance
- The Cell Wall: Structure, Composition, Functions, and Evolutionary Significance
- Endomembrane System: A Detailed Exploration of the Cellular Machinery of Life
- Mitochondria: A Comprehensive Guide to Their Structure and Functions
- The Cell Envelope: The Protective Shield of Prokaryotic Cells
- Ribosomes: Definition, Structure, and Functions
- Endoplasmic Reticulum: Structure, Types, and Functions Explained
- The Golgi Apparatus: Structure, Functions, and Cellular Importance
- Cytoplasm and Nucleus: Structure, Functions, Differences, and Examples
- What Is Cell Signaling? Structure, Types, and Functions Explained
- Three Main Parts of a Nucleotide: Structure and Functions
- Enzymes: Definition, Structure, Classification, and Functions
- DNA: Structure, Function, Discovery, and Modern Applications
- DNA Replication: Steps, Process, and Genetic Importance
- Properties of DNA: Structure, Physical, and Chemical Characteristics
- DNA Packaging: How DNA Fits into the Nucleus of a Cell
- R-Factors and Plasmids: How Bacteria Develop Antibiotic Resistance
- Chromosomes: Structure, Types, Functions, and Role in Genetics
- Genetic Material: DNA, RNA, and Their Role in Heredity and Evolution
- Deoxyribose and Ribose: Structure and Function in DNA and RNA
- Differences Between Genes and DNA: Definitions, Structures, and Functions
- RNA: Definition, Structure, Types, Functions, and Its Role in Life
Frequently Asked Questions
FAQ 1: What is RNA and why is it important in the body?
RNA, or ribonucleic acid, is a crucial molecule found in all living cells, playing a central role in how genetic information is used to sustain life. It acts as a messenger that carries instructions from DNA to produce proteins, which are essential for nearly every function in the body, from building tissues to fighting infections. Unlike DNA, which stores genetic information, RNA is typically single-stranded and more versatile, allowing it to perform multiple tasks like protein synthesis, gene regulation, and even acting as an enzyme.
RNA’s importance lies in its ability to bridge the gap between DNA’s genetic code and the proteins that carry out cellular tasks. This process is part of the central dogma of molecular biology, where DNA is transcribed into RNA, and RNA is translated into proteins. Without RNA, cells couldn’t produce the proteins needed for growth, repair, or responding to environmental changes. For example, when your body needs to fight a virus, RNA helps create the proteins that form antibodies.
Beyond protein synthesis, RNA also regulates gene expression, ensuring that genes are turned on or off at the right time. This makes RNA vital for development, immune responses, and maintaining cellular balance. Recent advances, like mRNA vaccines, highlight RNA’s role in modern medicine, showing its potential to address diseases by instructing cells to produce specific proteins.
FAQ 2: How is RNA different from DNA?
RNA and DNA are both nucleic acids, but they differ in structure, function, and location within cells, each tailored to specific roles. DNA, or deoxyribonucleic acid, is a double-stranded molecule that forms a stable helix, making it ideal for storing genetic information long-term. RNA, on the other hand, is usually single-stranded, allowing it to fold into unique shapes that enable diverse functions like protein synthesis and gene regulation.
A key structural difference is the sugar in their backbones. DNA contains deoxyribose, which lacks an oxygen atom, making it more stable. RNA has ribose, with an extra oxygen that makes it less stable but more flexible. Another difference is in their bases: DNA uses adenine (A), guanine (G), cytosine (C), and thymine (T), while RNA replaces thymine with uracil (U), which pairs with adenine during processes like transcription.
Functionally, DNA resides in the cell nucleus (in eukaryotes) and serves as the genetic blueprint, while RNA is produced in the nucleus but works mainly in the cytoplasm, carrying out tasks like translating genetic code into proteins. For instance, in a muscle cell, RNA ensures the instructions for muscle proteins are delivered from DNA to the protein-making machinery. These differences allow DNA to store information reliably while RNA acts dynamically to execute it.
FAQ 3: What are the main types of RNA and their functions?
RNA comes in several types, each with specialized roles in cells. The three primary types involved in protein synthesis are messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA), but there are also other important types like microRNA (miRNA) and small nuclear RNA (snRNA), each contributing to cellular processes.
mRNA carries genetic instructions from DNA to the ribosomes, the cell’s protein factories. It acts like a blueprint, specifying the order of amino acids to build a protein. For example, in insulin production, mRNA delivers the gene’s code to create the insulin protein. tRNA serves as an adapter, matching specific amino acids to their corresponding codes on mRNA, ensuring accurate protein assembly. rRNA forms the core of ribosomes and catalyzes the formation of peptide bonds between amino acids, making it essential for protein synthesis.
Other RNAs, like miRNA, regulate gene expression by binding to mRNA and preventing protein production, which is critical in processes like embryonic development. snRNA helps splice mRNA to remove unnecessary parts, ensuring only the correct instructions are used. These diverse roles highlight RNA’s versatility in maintaining cellular function and responding to environmental needs.
FAQ 4: How does RNA contribute to protein synthesis?
Protein synthesis is a two-step process—transcription and translation—where RNA plays a starring role. In transcription, DNA’s genetic code is copied into messenger RNA (mRNA) in the nucleus. This mRNA carries the code to the cytoplasm, where translation occurs at the ribosomes, the cell’s protein-making factories.
During translation, ribosomal RNA (rRNA) forms the structural and catalytic core of ribosomes, linking amino acids together to form proteins. Transfer RNA (tRNA) brings the correct amino acids to the ribosome by matching its anticodon to mRNA’s codons. For example, if mRNA has a codon for the amino acid glycine, a tRNA carrying glycine binds to it, ensuring the protein is built correctly.
This process is vital for life, as proteins perform tasks like oxygen transport (hemoglobin) or digestion (enzymes). RNA’s precise coordination ensures that genetic instructions are accurately translated into functional proteins, allowing cells to grow, repair, and respond to signals like hormones.
FAQ 5: What are ribozymes, and why are they significant?
Ribozymes are RNA molecules that act as enzymes, catalyzing chemical reactions in cells. Discovered in the 1980s by Sidney Altman and Thomas Cech, who won a Nobel Prize for their work, ribozymes challenged the belief that only proteins could be enzymes. Unlike DNA, RNA’s single-stranded nature and ribose sugar allow it to fold into complex shapes, enabling catalytic activity.
The most significant ribozyme is found in ribosomal RNA (rRNA) within ribosomes, where it catalyzes peptide bond formation during protein synthesis. Other ribozymes, like small nuclear RNAs (snRNAs), help splice mRNA, ensuring it’s ready for translation. For instance, in gene expression, snRNAs remove introns (non-coding regions) from mRNA, refining the genetic message.
Ribozymes are significant because they support the RNA world hypothesis, suggesting RNA was the first molecule of life, capable of both storing genetic information and catalyzing reactions. Today, researchers engineer ribozymes for medical applications, like targeting viral RNAs in diseases such as hepatitis, showcasing their potential in biotechnology.
FAQ 6: What is the RNA world hypothesis?
The RNA world hypothesis proposes that life on Earth began with RNA as the primary molecule, before DNA or proteins evolved. This idea, suggested in the 1980s, argues that RNA’s ability to store genetic information and act as a catalyst (via ribozymes) made it the ideal candidate for early life forms.
In this ancient world, RNA molecules could have self-replicated and performed chemical reactions, driving the evolution of simple life. Evidence includes the existence of ribozymes in modern cells, like those in ribosomes, and experiments showing that RNA nucleotides can form under prebiotic conditions. Over time, DNA likely emerged for stable genetic storage, and proteins took over most catalytic roles due to their efficiency.
This hypothesis is significant because it offers a glimpse into life’s origins, suggesting a simpler, RNA-based system preceded complex cells. It also inspires research into synthetic biology, where scientists create self-replicating RNAs to study early life or develop new therapies.
FAQ 7: How is RNA used in mRNA vaccines?
mRNA vaccines, like those developed for COVID-19, use synthetic messenger RNA (mRNA) to instruct cells to produce proteins that trigger an immune response. These vaccines deliver mRNA encoding a harmless piece of a virus, such as the spike protein of SARS-CoV-2, into cells. Once inside, the mRNA is translated by ribosomes to produce the viral protein, which the immune system recognizes and learns to fight.
Unlike traditional vaccines, mRNA vaccines don’t use live or weakened viruses, making them safer and faster to develop. The mRNA is temporary, breaking down after translation, and doesn’t alter the cell’s DNA. For example, when you receive an mRNA vaccine, your cells produce the spike protein, prompting your immune system to create antibodies without causing infection.
This technology, advanced in 2020, has revolutionized vaccine development, offering rapid responses to pandemics. As of 2025, mRNA vaccines are being explored for diseases like influenza, Zika, and even cancer, where they could train the immune system to target tumor cells.
FAQ 8: What role does RNA play in gene regulation?
RNA is a key player in gene regulation, controlling when and how genes are expressed to produce proteins. While messenger RNA (mRNA) carries the code for proteins, other RNAs, like microRNA (miRNA) and long non-coding RNA (lncRNA), fine-tune gene activity to ensure cells function properly.
miRNAs are short RNAs that bind to mRNA, either blocking translation or causing mRNA degradation, thus reducing protein production. For instance, in cancer, miRNAs might suppress tumor-suppressor genes, promoting uncontrolled cell growth. lncRNAs act as scaffolds, guiding proteins to specific DNA regions to turn genes on or off, critical in processes like X-chromosome inactivation in females.
Another mechanism is RNA interference, where small interfering RNAs (siRNAs) silence genes by targeting specific mRNAs for destruction. This is vital in defending against viruses, as siRNAs can degrade viral RNA. These regulatory roles ensure precise control over cellular processes, from development to stress responses, and their disruption can lead to diseases like cancer or neurological disorders.
FAQ 9: What are RNA viruses, and how do they work?
RNA viruses use RNA as their genetic material instead of DNA, relying on it to replicate and infect host cells. Examples include influenza, HIV, and SARS-CoV-2. Their RNA genomes can be single-stranded or double-stranded, and they often mutate rapidly due to RNA’s less stable nature, making them challenging to combat.
Single-stranded RNA viruses are either positive-sense, where the RNA acts directly as mRNA to produce viral proteins, or negative-sense, requiring transcription into mRNA first. For instance, HIV, a positive-sense RNA virus, reverse-transcribes its RNA into DNA, integrating it into the host’s genome to produce more virus particles.
RNA viruses hijack the host’s cellular machinery, using ribosomes to translate their RNA into proteins that build new viruses or evade the immune system. Viroids, a related group, are RNA molecules that infect plants without coding proteins, relying entirely on host enzymes for replication. RNA viruses’ adaptability drives their evolution, but it also fuels research into RNA-based therapies, like siRNA drugs, to target them.
FAQ 10: How is RNA research advancing modern medicine?
RNA research is transforming medicine, particularly through therapies and diagnostics. The success of mRNA vaccines for COVID-19 in 2020 demonstrated RNA’s potential to address diseases quickly and safely. These vaccines use synthetic mRNA to instruct cells to produce antigens, training the immune system without using live pathogens.
Beyond vaccines, RNA interference (RNAi) therapies, like patisiran for amyloidosis, use small interfering RNAs (siRNAs) to silence harmful genes, offering hope for genetic disorders. CRISPR-Cas13, an RNA-editing tool, targets RNA transcripts for precise gene regulation without altering DNA, useful for temporary interventions.
RNA research also advances diagnostics. The epitranscriptome, or chemical modifications on RNA, is being studied to detect diseases like cancer early, as these modifications affect RNA stability and function. As of 2025, clinical trials explore RNA therapies for heart disease, neurodegenerative disorders, and personalized cancer vaccines, promising a future where RNA-based treatments are commonplace, leveraging RNA’s versatility to improve health outcomes.
FAQ 11: What are the latest advancements in RNA research as of 2025?
As of September 2025, RNA research has seen remarkable progress, building on the foundations laid by earlier discoveries in molecular biology. Scientists are exploring new ways RNA can be harnessed for medical treatments, diagnostics, and even understanding fundamental life processes. One major area of advancement is in RNA therapeutics, where innovations extend beyond the well-known mRNA vaccines.
For instance, researchers have developed more sophisticated RNA-based drugs that target specific diseases with greater precision, such as using RNA interference to silence faulty genes in conditions like cancer and genetic disorders. This year alone, clinical trials have shown promising results in using RNA to treat rare diseases, with some therapies gaining regulatory approval faster than ever before.
Another exciting development is in the field of single-cell RNA sequencing and epitranscriptomics, which allow scientists to map RNA modifications at an unprecedented level. These techniques reveal how chemical changes to RNA molecules affect their stability and function, providing insights into complex diseases like neurodegenerative disorders. In 2025, breakthroughs in high-throughput sequencing have made it possible to analyze RNA from individual cells in real time, helping to uncover how RNA regulates gene expression during development or in response to environmental stresses. This has implications for personalized medicine, where treatments could be tailored based on a patient’s unique RNA profile.
RNA’s role in cancer research has also advanced significantly. New studies focus on RNA-based vaccines and therapies that target tumor-specific mutations, showing improved efficacy in clinical settings. For example, combination therapies that use RNA to boost immune responses against cancer cells have entered late-stage trials, potentially revolutionizing oncology. Additionally, the integration of artificial intelligence in predicting RNA structures has accelerated drug discovery, allowing researchers to design RNA molecules that fold in specific ways to interact with disease-causing proteins.
Looking ahead, conferences like RNA Horizons 2025 have highlighted emerging areas such as RNA editing tools, which could correct genetic errors at the RNA level without altering DNA. This approach holds promise for treating inherited diseases safely. Overall, these advancements underscore RNA’s versatility, from diagnostic tools that detect diseases early to therapeutics that offer new hope for chronic conditions, marking 2025 as a pivotal year in RNA science.
FAQ 12: How does RNA folding influence its function?
RNA folding is a dynamic process that shapes how this molecule performs its essential roles in cells, from protein synthesis to gene regulation. The way RNA folds determines its stability, interactions with other molecules, and overall functionality, making it a key factor in biological processes.
- Formation of Secondary Structures: RNA molecules fold into structures like hairpins, loops, and stems through base pairing, where adenine pairs with uracil and guanine with cytosine. These structures are crucial for stability and allow RNA to bind to proteins or other RNAs, influencing functions such as catalysis in ribozymes. For example, in ribosomal RNA, folding creates the active site for protein assembly, ensuring efficient translation.
- Impact on Gene Regulation: Folded RNA can act as switches, changing shape in response to environmental cues like temperature or metabolites. This conformational shift can expose or hide binding sites, regulating gene expression. In bacteria, riboswitches fold to sense small molecules and control transcription, demonstrating how folding directly affects cellular responses.
- Role in Cotranscriptional Folding: As RNA is transcribed from DNA, it begins folding immediately, a process called cotranscriptional folding. This timing influences the final structure and function, potentially guiding alternative splicing or preventing misfolding. Disruptions here can lead to diseases, highlighting folding’s importance in maintaining cellular health.
- Tertiary Structures and Functionality: Beyond secondary folds, RNA can form complex 3D shapes stabilized by interactions like metal ions. These tertiary structures enable advanced functions, such as in transfer RNA’s L-shape, which is vital for accurate amino acid delivery during protein synthesis. Misfolding can impair this, leading to errors in protein production.
- Therapeutic Implications: Understanding RNA folding has led to drug designs that target specific folds, like in antiviral therapies that disrupt viral RNA structures. Advances in computational modeling now predict folds accurately, aiding in the development of RNA-based medicines.
- Evolutionary Perspective: RNA folding’s flexibility likely played a role in early life, allowing primitive RNA to catalyze reactions and store information, supporting the RNA world hypothesis. Today, this adaptability continues to drive RNA’s diverse roles in modern organisms.
FAQ 13: What is the role of RNA in epigenetics?
RNA plays a pivotal part in epigenetics, the study of changes in gene expression without altering the DNA sequence itself. Through various mechanisms, RNA molecules help regulate how genes are turned on or off, influencing traits from development to disease susceptibility. For instance, non-coding RNAs like long non-coding RNAs can interact with chromatin, the complex of DNA and proteins, to modify its structure and accessibility. This allows cells to respond to environmental signals without permanent genetic changes, a process essential for adaptation and survival.
In more detail, small RNAs such as microRNAs contribute to epigenetic regulation by binding to messenger RNA and preventing protein production, effectively silencing genes. This post-transcriptional control adds a layer of fine-tuning to gene expression, analogous to how histone modifications or DNA methylation work. Research shows that these RNA-mediated changes can be heritable, passing from one generation to the next in some cases, like in plants where small RNAs transmit stress responses to offspring.
Epigenetic roles of RNA extend to chromatin remodeling, where RNAs guide enzymes to specific genomic locations. For example, in X-chromosome inactivation, a long non-coding RNA coats one X chromosome in females, leading to its silencing and ensuring proper dosage compensation. Disruptions in these RNA functions can lead to disorders, including cancers where aberrant RNA modifications alter gene expression patterns.
Furthermore, RNA-binding proteins interact with RNAs to influence epigenetic marks, creating a network that integrates RNA into broader regulatory systems. Recent studies highlight how circular RNAs, a type of non-coding RNA, participate in epigenetic processes during neurodevelopment, potentially affecting brain function and behavior. As research progresses, understanding RNA’s epigenetic roles could unlock new therapies for age-related diseases or developmental conditions.
FAQ 14: How is RNA involved in viral infections?
RNA’s involvement in viral infections is multifaceted, from serving as the genetic material for many viruses to playing key roles in host defense mechanisms. Below is a detailed table outlining various aspects of this involvement, including viral strategies and host responses.
| Aspect of Involvement | Description | Examples | Impact on Infection |
|---|---|---|---|
| Viral Genome | Many viruses use RNA as their genetic blueprint, allowing quick replication inside host cells. | Influenza, HIV, SARS-CoV-2 | Enables rapid mutation and adaptation, making vaccines challenging to develop. |
| Host Immune Response | Cellular RNA interference pathways degrade viral RNA to prevent spread. | RNA interference in plants and animals | Limits viral replication; disruptions can lead to severe infections. |
| Viral Evasion Tactics | Viruses encode proteins that interfere with host RNA processing or sensing. | Herpesviruses manipulating host RNA decay | Allows viruses to hijack cellular machinery for their benefit. |
| Long Non-Coding RNAs | These host RNAs regulate immune genes during infection, either promoting or suppressing responses. | lncRNAs in COVID-19 response | Can exacerbate inflammation or aid in viral clearance. |
| Circular RNAs | Viral-encoded circular RNAs act as sponges for host microRNAs, altering gene expression. | Epstein-Barr virus circRNAs | Promotes viral persistence by disrupting host defenses. |
| MicroRNAs | Host microRNAs target viral RNAs for degradation, while viruses can produce their own to manipulate hosts. | miRNAs in hepatitis C | Influences disease progression and potential therapeutic targets. |
| RNA-Binding Proteins | These proteins bind viral RNAs to control trafficking and replication. | MKRN2 in influenza | Affects viral mRNA export from nucleus to cytoplasm. |
| Innate Immunity Activation | Detection of viral RNA triggers interferon production and antiviral states. | RIG-I sensing double-stranded RNA | Initiates broad antiviral defenses in macrophages and dendritic cells. |
This table illustrates RNA’s central role in the battle between viruses and hosts, highlighting opportunities for antiviral drug development.
FAQ 15: What are non-coding RNAs, including their types, importance, and functions?
Non-coding RNAs are RNA molecules that do not translate into proteins but play critical regulatory roles in cells. They make up the majority of the transcriptome and are essential for maintaining cellular balance, responding to stresses, and influencing development.
- Definition and Importance: Unlike coding RNAs like mRNA, non-coding RNAs orchestrate gene expression, chromatin structure, and RNA processing. Their importance lies in adding layers of control to the genome, allowing complex organisms to evolve without expanding protein-coding genes. Dysregulation can lead to diseases like cancer or neurological disorders.
- Housekeeping Types: These include ribosomal RNA (rRNA) and transfer RNA (tRNA), which are vital for protein synthesis. rRNA forms the ribosome’s core, while tRNA delivers amino acids, ensuring basic cellular functions run smoothly.
- Regulatory Types – MicroRNAs (miRNAs): Short RNAs that bind to mRNA to inhibit translation or cause degradation. They fine-tune gene expression, crucial in development and immunity. For example, miRNAs regulate cell differentiation.
- Long Non-Coding RNAs (lncRNAs): Longer than 200 nucleotides, they modulate chromatin and transcription. Some act as scaffolds for protein complexes, influencing epigenetics and disease progression.
- Circular RNAs (circRNAs): Closed-loop structures resistant to degradation, functioning as miRNA sponges or regulators. They’re important in brain function and cancer.
- Small Interfering RNAs (siRNAs): Involved in RNA interference, silencing genes by targeting mRNA. Key in antiviral defenses and gene therapy.
- Functions in Health and Disease: Non-coding RNAs regulate everything from embryonic development to stress responses. In medicine, they’re biomarkers and therapeutic targets, emphasizing their broad significance.
FAQ 16: How does RNA editing work, including mechanisms and examples?
RNA editing is a post-transcriptional process where specific nucleotides in RNA are altered, expanding the diversity of proteins without changing the DNA code. This mechanism increases genetic flexibility, allowing cells to produce multiple protein variants from a single gene. The primary mechanisms include substitution, insertion, and deletion of bases, each catalyzed by specialized enzymes.
Substitution editing, the most common, involves chemical changes like deamination. For adenine-to-inosine editing, ADAR enzymes convert adenine to inosine, which is read as guanine during translation. This occurs in brain tissues, affecting neurotransmitter receptors and influencing neural function. In cytosine-to-uracil editing, APOBEC enzymes perform the conversion, seen in antiviral responses where it mutates viral RNA to halt replication.
Insertion and deletion editing are prominent in certain organisms. In trypanosomes, guide RNAs direct the addition or removal of uridines in mitochondrial mRNA, correcting frameshifts to produce functional proteins. This extensive editing can alter up to half the sequence, showcasing RNA’s adaptability in parasites.
Examples abound in human health: RNA editing of the glutamate receptor mRNA changes a single amino acid, reducing calcium permeability and preventing excitotoxicity in neurons. Aberrant editing links to diseases like epilepsy or cancer, where over-editing promotes tumor growth. Therapeutically, harnessing RNA editing could correct genetic mutations, offering precise treatments without genome editing risks.
FAQ 17: What is the role of RNA in plant biology?
RNA’s roles in plant biology are diverse, from regulating growth to defending against pests. The table below details key functions, structures, and examples in plants.
| Role | Description | Key RNA Types Involved | Examples in Plants |
|---|---|---|---|
| Gene Regulation | RNA controls when and where genes are expressed, adapting to light, water, or nutrients. | MicroRNAs, long non-coding RNAs | miRNAs regulate flowering in Arabidopsis. |
| Stress Response | Helps plants withstand drought, salinity, or cold by altering gene expression. | Small interfering RNAs | siRNAs activate defenses against heat stress. |
| Development | Guides organ formation, root growth, and seed germination. | Transfer RNA, ribosomal RNA | tRNA ensures protein synthesis during growth. |
| Defense Against Pathogens | RNA interference silences viral or fungal genes. | Circular RNAs, viroid RNAs | circRNAs sponge miRNAs in pathogen resistance. |
| Secondary Metabolite Production | Regulates biosynthesis of compounds like alkaloids for protection. | Long non-coding RNAs | lncRNAs enhance terpenoid production under stress. |
| Epigenetic Modifications | Influences heritable changes without DNA alterations. | Small RNAs | Small RNAs mediate DNA methylation in response to environment. |
| Intercellular Communication | Mobile RNAs signal between cells for coordinated responses. | Messenger RNA | mRNAs travel via phloem to distant tissues. |
| Structural Functions | Forms scaffolds for cellular processes. | Ribosomal RNA | rRNA in chloroplasts supports photosynthesis. |
This table highlights RNA’s integral contributions to plant survival and adaptation.
FAQ 18: What are therapeutic applications of RNA beyond vaccines?
RNA therapeutics have expanded far beyond vaccines, offering innovative treatments for various diseases by targeting genetic and molecular pathways.
- RNA Interference for Gene Silencing: Uses small interfering RNAs to knock down disease-causing genes, effective in treating conditions like amyloidosis or high cholesterol. Approved drugs like patisiran demonstrate this by reducing protein buildup in nerves.
- Antisense Oligonucleotides: These bind to mRNA to prevent faulty protein production, used in spinal muscular atrophy and Duchenne muscular dystrophy. They correct splicing errors, improving muscle function.
- RNA Editing Technologies: Tools like CRISPR-Cas13 edit RNA transcripts temporarily, ideal for reversible corrections in genetic diseases without DNA changes. In 2025, trials target cystic fibrosis mutations.
- Protein Replacement Therapy: Synthetic mRNA delivers instructions for missing proteins, treating enzyme deficiencies in metabolic disorders like Fabry disease.
- Cancer Therapies: RNA-based drugs target oncogenic pathways, with siRNAs silencing tumor growth factors. Combined with immunotherapy, they enhance treatment outcomes.
- Cardiac and Regenerative Medicine: mRNA promotes heart tissue repair post-infarction or aids bone regeneration, showing promise in clinical studies.
- Neurological Applications: RNA therapeutics cross the blood-brain barrier to treat Alzheimer’s by reducing amyloid plaques or Huntington’s by silencing mutant genes.
FAQ 19: How does RNA play a role in aging and longevity?
RNA influences aging and longevity through regulatory networks that control cellular maintenance, stress responses, and gene expression over time. As organisms age, changes in RNA processing, such as altered splicing or modifications, contribute to declining cellular function.
For example, long non-coding RNAs modulate pathways like insulin signaling, which are linked to lifespan extension in model organisms. Studies show that enhancing certain RNAs can mimic calorie restriction, a known longevity promoter, by improving metabolic efficiency and reducing oxidative damage.
Circular RNAs, stable molecules that accumulate with age, act as miRNA sponges, affecting gene regulation. In older tissues, their dysregulation leads to inflammation or neurodegeneration, accelerating aging. Conversely, some circular RNAs promote longevity by stabilizing beneficial pathways, as seen in worms where specific circRNAs extend lifespan through stress resistance.
RNA helicases, enzymes that unwind RNA, also play roles in aging by ensuring proper folding and translation. Mutations in these can shorten lifespan, while boosting their activity delays age-related decline. Recent experiments injecting RNA molecules into mice restored cellular youthfulness, increasing lifespan and vitality, suggesting therapeutic potential.
Overall, RNA’s involvement in epigenetics and quality control underscores its importance in longevity research, with implications for anti-aging interventions.
FAQ 20: What is the evolutionary significance of RNA?
RNA’s evolutionary significance stems from its proposed central role in early life, as outlined in the RNA world hypothesis. This theory suggests that RNA was the first molecule to both store genetic information and catalyze reactions, predating DNA and proteins. In primitive environments, self-replicating RNA could have driven the emergence of life, with its versatility allowing simple chemical systems to evolve complexity.
As life progressed, RNA’s functions diversified, facilitating the transition to DNA-based genomes for stability while retaining catalytic roles in ribozymes. This shift likely improved efficiency, with RNA adapting to regulatory tasks in modern cells. Evidence from retrotransposons shows RNA’s influence on genome evolution, inserting sequences that drive diversity.
In multicellular organisms, RNA enabled sophisticated gene regulation, supporting the leap from single cells to complex life. Its conservation across species highlights its foundational importance in evolution.
Acknowledgement
The Examsmeta website expresses its sincere gratitude to the following reputable sources for providing valuable insights and scientific data that greatly enriched the article “RNA: Definition, Structure, Types, Functions, and Its Role in Life.” Their comprehensive resources ensured the accuracy and depth of the information presented, making this article a reliable guide for readers seeking to understand RNA’s multifaceted roles in biology and medicine.
Below are the key sources acknowledged for their contributions:
- Nature (www.nature.com): For detailed studies on RNA structure, function, and advancements in RNA-based therapeutics, including insights into epitranscriptomics and RNA editing.
- ScienceDirect (www.sciencedirect.com): For peer-reviewed articles on RNA’s roles in gene regulation, viral infections, and plant biology, offering a robust scientific foundation.
- PubMed (pubmed.ncbi.nlm.nih.gov): For access to primary research on RNA’s involvement in epigenetics, aging, and therapeutic applications, ensuring up-to-date medical perspectives.
- National Human Genome Research Institute (www.genome.gov): For clear explanations of RNA’s role in the central dogma and its significance in genetic processes.
- The Conversation (theconversation.com): For accessible insights into RNA’s enzymatic capabilities and evolutionary significance, particularly the RNA world hypothesis.

