Imagine a world inside your cells where tiny chemical messengers don’t need to step outside to get their job done. They stay right there, whispering instructions that can change everything from how your heart beats to whether a tumor grows out of control. That’s the magic of intracrine signaling – a hidden conversation happening deep within our bodies.
For decades, scientists have focused on how hormones travel long distances or chat with neighboring cells, but intracrine signaling flips the script. It’s all about action at home, inside the very cell that makes these signals. This internal dialogue isn’t just a curiosity; it’s a powerhouse that influences everything from immune defenses to metabolic balance.
In this deep dive, we’ll explore what makes intracrine signaling tick, how it differs from the usual suspects in cell communication, real-world examples from everyday health to serious illnesses, and why it’s sparking a revolution in medicine. Buckle up – there’s a lot to unpack.
Table of Contents
What Exactly is Intracrine Signaling?
Intracrine signaling is like a cell’s private pep talk. When a hormone or growth factor gets produced inside a cell, it doesn’t always pack up and leave through the bloodstream or diffuse to nearby buddies. Instead, it sticks around, binding to receptors right there in the cytoplasm or even the nucleus. This setup lets the cell fine-tune its own behavior without outside interference, regulating things like gene expression, cell growth, and survival on the fly.
Think of it this way: in traditional signaling, messages are shouted across a room or sent via mail. But intracrine? It’s a text to yourself. The term was first tossed around in the 1980s to describe peptides that either work within their birth cell or get pulled back in after a quick jaunt outside. Over time, it’s grown to cover a broader crew of molecules – hormones, growth factors, you name it – as long as they trigger action from the inside out. This isn’t some rare glitch; it’s a fundamental way cells stay in control, especially in tissues that need quick, precise responses like the heart or immune system.
One cool twist: not all intracrines are total homebodies. Some get secreted to nudge neighbors in a paracrine fashion but still pull double duty inside their original cell. This flexibility makes intracrine signaling a Swiss Army knife for biology, adapting to whatever the body throws at it.
A Quick History: From Niche Idea to Scientific Staple
The story of intracrine signaling starts in the labs of the early 1980s, when researchers poking around with angiotensin II – a key player in blood pressure control – noticed something odd. This peptide wasn’t just hanging out on cell surfaces; it was showing up in the nucleus and mitochondria, places no one expected it to be. That sparked the birth of “intracrinology,” the study of these internal escapades. By the mid-90s, evidence piled up: receptors for these signals were popping up inside cells, and blocking them had effects that surface-only drugs couldn’t touch.
Fast forward three decades, and intracrinology has exploded. What began with a few peptides has ballooned to include growth factors like VEGF and FGF2, cytokines, even bits of enzymes. Key breakthroughs? Discovering how these molecules hitch rides on internal transport systems, like exosomes or nanotubes, to zip around without ever leaving the cell. We’ve seen feed-forward loops where a signal ramps up its own production, creating self-sustaining cycles that drive everything from heart development to cancer growth. Today, with tools like super-resolution microscopy, we’re mapping these paths in real time, revealing a bustling intracellular highway system.
This evolution hasn’t been smooth – skeptics clung to the old “surface receptor only” model for years. But observational data, from animal models to human tissues, has sealed the deal. Intracrine signaling isn’t a footnote; it’s rewriting textbooks on how hormones really work.
How Does It Stack Up? Intracrine vs. Other Cell Signaling Types
Cells have a whole toolkit for chatting, and intracrine is just one player. To get why it’s special, let’s break down the main types side by side. Endocrine signaling is the long-haul traveler, paracrine the local gossip, autocrine the self-motivator – and intracrine? The ultimate introvert, handling business solo without ever airing its laundry.
Here’s a handy comparison to visualize the differences:
| Signaling Type | Distance of Action | Key Features | Classic Examples | Receptor Location |
|---|---|---|---|---|
| Endocrine | Long-range (via bloodstream to distant organs) | Hormones travel system-wide for broad effects like growth or stress response. | Insulin from pancreas regulating blood sugar in muscles; thyroid hormones boosting metabolism everywhere. | Mostly cell surface, some intracellular. |
| Paracrine | Short-range (diffuses to nearby cells) | Local tweaks, like coordinating tissue repair without whole-body alerts. | Growth factors in wound healing signaling to adjacent skin cells; neurotransmitters firing across synapses. | Primarily cell surface. |
| Autocrine | Same cell (secreted then rebounds) | Self-stimulation for amplification, often in immune or cancer cells. | Tumor cells releasing their own growth signals to fuel unchecked division; T-cells boosting their own activation. | Cell surface, with some internalization. |
| Intracrine | Strictly intracellular (no secretion needed) | Autonomous control, targeting nucleus or cytoplasm for direct gene tweaks. | Angiotensin II regulating heart cell growth from inside; VEGF keeping stem cells alive without leaving the cell. | Cytoplasm or nucleus. |
This table highlights how intracrine skips the export step entirely, making it faster and more private. While the others rely on diffusion or circulation – which can dilute signals or invite eavesdroppers – intracrine keeps everything locked down. In practice, these aren’t silos; they overlap. A molecule might start paracrine but end up intracrine after uptake. That’s the beauty – cells mix and match for precision.
The Mechanisms Behind Intracrine Magic
Diving deeper, how does this internal signaling actually happen? It starts with synthesis: the cell whips up its messenger, often from local precursors, bypassing the need for imports. Then comes trafficking – no highways here, just clever shuttles. Many intracrines use nuclear localization signals (NLS), protein tags that flag them for nucleus delivery. Others hitch onto receptors that get swallowed via endocytosis, only to resurface inside.
Once paired, the duo activates pathways. Bind to a nuclear receptor? Boom – gene transcription fires up, cranking out proteins for growth or repair. Hit cytoplasmic spots? You might tweak metabolism or zap survival signals. A standout mechanism is the feed-forward loop: the signal boosts its own maker, creating a snowball effect. Take angiotensin II: it binds nuclear AT-1 receptors, ramps up renin and angiotensinogen genes, and sustains the cycle – perfect for chronic tweaks like in hypertension.
But it’s not all smooth sailing. Regulation is key – enzymes degrade extras, while stress or nutrients dial levels up or down. In high-glucose environments, like diabetes, this amps up, linking intracrine glitches to disease. Tools like exosomes add layers, ferrying signals between organelles without full export. It’s a ballet of molecules, choreographed for efficiency.
Intracrine Signaling in Normal Physiology
Intracrine signaling isn’t just for crises; it’s the unsung hero of daily life, keeping tissues humming. Let’s spotlight some stars.
In the heart, angiotensin II plays conductor. Produced locally in cardiac cells, it binds internal receptors to tweak electrical signals and prevent arrhythmias. Without this, beats could falter under stress. Similarly, during embryonic development, dynorphin B sparks a loop that nudges stem cells toward heart tissue, ensuring proper formation.
Over in the brain, VEGF-A keeps neural stem cells in the hippocampus alive and kicking. These cells hug blood vessels, using internal VEGF to dodge exhaustion and support learning. Knock it out? Memory dips, and neurodegeneration looms. In bones, VEGF again shines, pushing osteoblasts to build strong trabeculae while curbing fat buildup – a win for warding off osteoporosis.
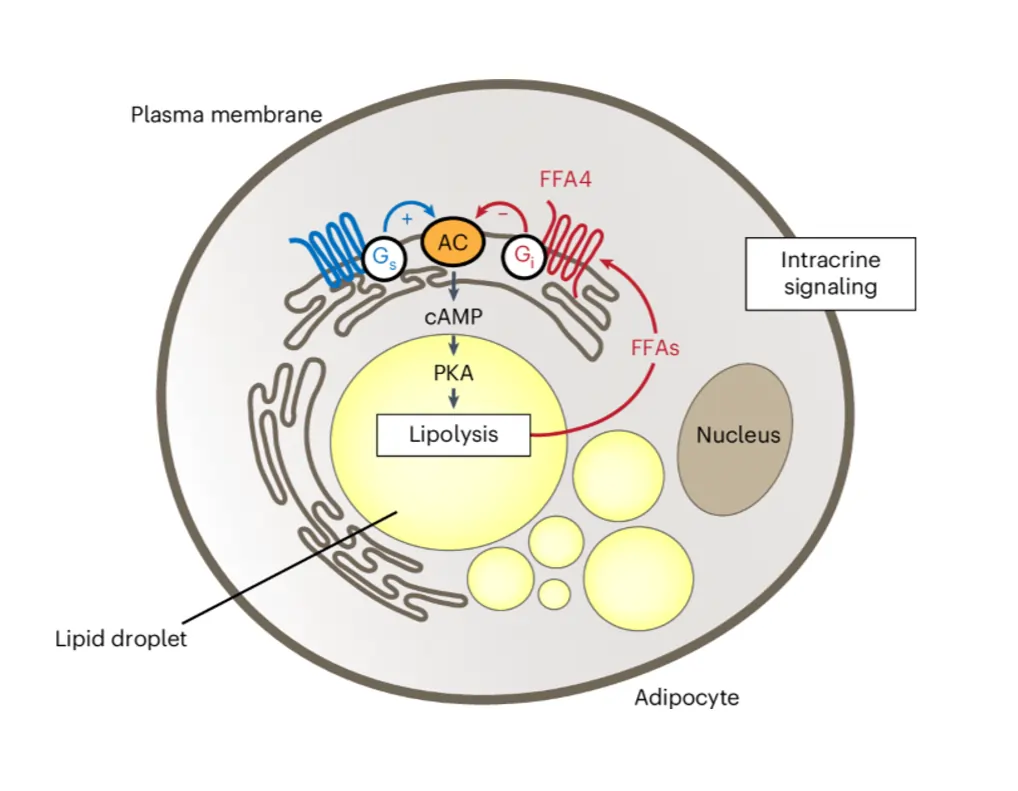
Immune cells? They’re intracrine pros. Macrophages churn out sex steroids like 17α-estradiol, which dials down inflammation by tweaking cytokine genes internally. This keeps responses balanced, not raging. In metabolism, adipocytes use FFA4 receptors on lipid droplets to fine-tune fat breakdown. Free fatty acids from lipolysis ping these internal sensors, slamming the brakes via cAMP drops – a neat feedback to avoid overload.
- Vascular Health: FGF2 and angiogenin team up in loops driving new vessel sprouts, with nuclear trafficking key for RNA synthesis.
- Stem Cell Maintenance: Homeodomain factors like PDX-1 get internalized, reprogramming cells to insulin producers for blood sugar control.
- Muscle Power: Intracrine androgens in skeletal fibers boost strength, tying into daily endurance.
These examples show intracrine as the cell’s autopilot, handling routine ops with quiet efficiency.
When Things Go Awry: Intracrine’s Dark Side in Disease
Flip the coin, and intracrine signaling can fuel trouble. In pathology, those self-sustaining loops turn rogue, amplifying harm.
Cancer steals the spotlight. In breast tumors, macrophages pump out estrogens via aromatase, fueling cell proliferation through internal estrogen receptors – a paracrine-intracrine hybrid that correlates with aggressiveness. VEGF-A goes villainous too: in colorectal cancer, internal loops keep cells alive despite chemo, hiking resistance. Knockdown? Apoptosis surges, and growth stalls. In renal cell carcinoma, intracrine androgens sustain tumors post-hormone therapy, explaining castration resistance.
Inflammation’s another hotspot. Rheumatoid arthritis synovial cells crank 16-hydroxyestrone, a mitogenic estrogen that ramps joint damage via NFκB tweaks. Immune dimorphisms shine here – post-trauma, male T-cells boost DHT for protection, while females up aromatase, risking autoimmunity.
Metabolic mayhem? Diabetic hearts overdrive angiotensin II intracrine, spiking mitochondrial stress and ROS, paving cardiomyopathy. In obesity, faulty FFA4 at lipid droplets lets lipolysis run wild, worsening insulin resistance.
- AML and Myeloma: Nuclear VEGFR2/1 drives unchecked blood cell growth, outpacing surface blockers.
- Prostate Cancer: De novo steroidogenesis via arachidonic acid loops evades therapies.
- Neurodegeneration: Exosome-trafficked prions mimic intracrine amplification in Alzheimer’s, seeding plaques.
These glitches underscore intracrine’s double-edged sword – vital for health, vicious in excess.
Charting New Therapies: Harnessing Intracrine for Healing
The therapeutic goldmine? Targeting intracrine paths directly. Traditional drugs hit surfaces; these go inside, potentially zapping resistance.
Consider cancer: Inhibiting angiogenin nuclear entry with neamine halts breast proliferation. For VEGF-driven tumors, cell-permeable kinase blockers like sunitinib outperform antibodies, shrinking skin cancers by 50% more in models. In colon cancer, disrupting internal VEGF-VEGFR1 slashes survival signals, boosting chemo kill rates.
Metabolic wins abound. Permeable FFA4 agonists could tame lipolysis in diabetes, curbing fatty acid floods. For immunity, tweaking macrophage steroidogenesis – say, via StAR modulators – might dial down RA inflammation without systemic side effects.
Cardio perks: Beyond ACE inhibitors, mitochondrial angiotensin blockers could prevent diabetic heart woes. Regenerative med? Cell-penetrating peptides from PDX-1 reprogram bile cells to insulin factories, sans viruses – safer for diabetes cures.
Here’s a table of promising targets:
| Disease Area | Intracrine Target | Mechanism | Potential Drug Approach | Expected Benefit |
|---|---|---|---|---|
| Cancer (Breast/Prostate) | Aromatase/Estrogen Receptors | Local estrogen loops fueling growth. | Enzyme inhibitors like letrozole, enhanced for uptake. | Reduced tumor estrogen, slower progression. |
| Colorectal Cancer | VEGF-VEGFR1 Complex | Kinase-independent survival signaling. | Internal tyrosine phosphatase activators. | Increased apoptosis, chemo synergy. |
| Rheumatoid Arthritis | 16-Hydroxyestrone Pathways | Mitogenic inflammation via NFκB. | Selective 5α-reductase blockers in synovium. | Lower joint damage, less cytokine storm. |
| Diabetes/Cardiomyopathy | Mitochondrial Angiotensin II | ROS generation in high-glucose states. | Mitochondria-targeted AT-1 antagonists. | Protected heart function, fewer arrhythmias. |
| Obesity/Metabolic Syndrome | FFA4 at Lipid Droplets | Dysregulated lipolysis feedback. | Permeable G_i/o agonists. | Balanced fat breakdown, better insulin sensitivity. |
| Neuroregeneration | VEGF in Hippocampal Stem Cells | Preventing NSC exhaustion. | Internal VEGF mimetics via exosomes. | Enhanced memory, slowed Alzheimer’s. |
This lineup shows intracrine’s edge: precision strikes at the source, minimizing off-target hits.
Looking Ahead: The Frontiers of Intracrine Research
The field’s buzzing. With CRISPR editing internal loops and AI modeling trafficking, we’re on the cusp of breakthroughs. Imagine designer peptides that slip in and reset cancer loops or metabolic dials. Challenges? Delivery – getting drugs inside without toxicity. But advances in nanoparticles and exosome hijacking are closing gaps.
Ethically, it’s thorny: tweaking self-signals could ripple system-wide. Yet, the payoff – personalized meds for resistant cancers or tailored immune boosts – is huge. As we map more molecules, like emerging roles in senescence, intracrine could redefine aging therapies.
Why Intracrine Matters to You and Me
Intracrine signaling isn’t abstract science; it’s the quiet force steering our biology. From keeping your immune system chill to nipping tumors in the bud, it proves cells are smarter than we thought – self-reliant powerhouses with internal wisdom. As research races forward, expect treatments that listen to these whispers, turning disease whispers into roars of recovery. Next time you feel your heart steady or a cut heal, thank the intracrine crew working overtime inside. It’s a reminder: the most profound changes often start small, right at home.
Frequently Asked Questions
FAQ 1: What is intracrine signaling and why is it important for understanding cellular health?
Intracrine signaling represents a fascinating way cells communicate with themselves, acting like an internal feedback system that keeps everything running smoothly without needing to send messages outside. At its heart, this process involves hormones or growth factors that are produced right inside a cell and then bind to receptors within that same cell, often in the cytoplasm or nucleus, to influence functions like gene expression, cell growth, and survival. Unlike more familiar types of signaling where molecules travel far or chat with neighbors, intracrine signaling stays completely contained, allowing for quick, precise control that’s essential for maintaining balance in tissues throughout the body.
This internal dialogue becomes especially crucial when you consider how it supports everyday health. For instance, in heart cells, local production of molecules like angiotensin II helps regulate electrical impulses to prevent irregular beats during stress. Without this self-talk, cells might overreact or fail to adapt, leading to broader issues like weakened heart function over time. Researchers have found that intracrine mechanisms are at play in everything from brain development to bone strength, making them a cornerstone of how our bodies stay resilient.
Diving a bit deeper, the beauty of intracrine signaling lies in its autonomy. Cells don’t wait for distant signals from the brain or bloodstream; they can ramp up or dial down their own activity based on immediate needs, such as during exercise when muscle cells boost their internal androgens for extra power. This efficiency not only conserves energy but also minimizes interference from other parts of the body, which is why disruptions here can ripple into diseases like diabetes or inflammation. As studies continue to uncover more about these hidden pathways, it’s clear that grasping intracrine signaling could unlock new ways to support cellular health and prevent common ailments.
FAQ 2: How does intracrine signaling compare to other types of cell signaling like endocrine and paracrine?
To really appreciate intracrine signaling, it’s helpful to see how it fits into the bigger picture of cell communication, where different strategies handle various ranges and speeds of interaction. While endocrine signaling sends hormones on long journeys through the blood to distant organs, paracrine keeps things local by diffusing to nearby cells, and autocrine loops back to the same cell after a brief secretion, intracrine skips the export step altogether for purely internal action. This comparison highlights why intracrine is uniquely suited for rapid, private adjustments that don’t risk dilution or unwanted eavesdropping.
Here’s a detailed breakdown in table form to clarify the distinctions:
| Signaling Type | Range of Action | Key Characteristics | Examples in Action | Receptor Sites |
|---|---|---|---|---|
| Endocrine | Long-distance (bloodstream to far-off tissues) | Slow but widespread effects, ideal for whole-body coordination like metabolism or stress responses. | Thyroid hormones traveling to every cell to rev up energy use; insulin from the pancreas hitting muscles to manage sugar levels. | Mainly surface, with some internal uptake. |
| Paracrine | Short-distance (to adjacent cells only) | Quick local tweaks for teamwork in tissues, without alerting the entire system. | Wound-site growth factors nudging skin cells to multiply and seal the gap; synaptic chemicals firing between neurons. | Predominantly cell surface. |
| Autocrine | Same cell (secreted then reabsorbed) | Self-boost for amplification, common in growing or defending cells. | Cancer cells secreting their own fuels to keep dividing wildly; activated immune cells ramping up their response. | Surface with internalization for deeper effects. |
| Intracrine | Strictly inside the cell (no leaving required) | Ultra-fast, autonomous control for precise internal tweaks like gene activation. | Angiotensin II inside heart cells steadying rhythms; VEGF keeping brain stem cells alive without export. | Cytoplasm or nucleus for direct impact. |
This setup shows intracrine as the most contained option, often overlapping with others for hybrid effects, like a secreted molecule getting re-internalized. Understanding these differences helps explain why targeting intracrine paths could offer more targeted therapies compared to broad endocrine blockers.
FAQ 3: What is the history behind the discovery of intracrine signaling?
The journey of intracrine signaling from a quirky observation to a core concept in biology spans over four decades, starting with puzzled researchers noticing that certain peptides seemed to defy the rules of classic hormone action. Back in the early 1980s, studies on angiotensin II, a molecule famous for blood pressure control, revealed it wasn’t just sticking to cell surfaces but sneaking into the nucleus and mitochondria to tweak genes directly. This unexpected behavior led to the coining of “intracrine” in 1987, marking the birth of intracrinology as a field dedicated to these internal escapades.
As the years rolled on, the 1990s brought a flood of evidence from labs worldwide, showing that growth factors like basic fibroblast growth factor (FGF2) and vascular endothelial growth factor (VEGF) followed similar paths, binding internal receptors to drive cell growth without ever leaving home. Skepticism lingered at first, with many clinging to the surface-receptor dogma, but by the early 2000s, advanced imaging tools confirmed these pathways in real time, solidifying intracrine’s role.
Key milestones in this evolution include:
- 1980s Foundations: Initial angiotensin II work sparks the intracrine hypothesis, challenging endocrine norms.
- 1990s Expansion: Discovery of nuclear receptors for peptides like endothelin, broadening the model to growth factors.
- 2000s Validation: Feed-forward loops identified, linking intracrine to chronic conditions like hypertension.
- 2010s Boom: Exosome trafficking revealed, showing how signals shuttle internally without secretion.
- 2020s Advances: Integration with CRISPR for editing loops, paving way for therapies in cancer and metabolism.
Today, with over 30 years under its belt, intracrinology continues to evolve, influencing how we view everything from immune responses to aging.
FAQ 4: What are the main mechanisms that drive intracrine signaling inside cells?
Intracrine signaling kicks off with the cell crafting its own messengers from local building blocks, ensuring no delay from external sources. These molecules, often peptides or steroids, then navigate via clever tags like nuclear localization signals that guide them to the nucleus for direct chats with DNA, flipping switches on genes for growth or repair. In the cytoplasm, they might latch onto receptors to tweak enzymes or metabolic pathways, creating ripples that sustain the cell’s daily grind.
Regulation keeps this in check, with breakdown enzymes clearing out extras and environmental cues like nutrient levels dialing production up or down. A standout feature is the self-amplifying loop, where the signal boosts its own factory, turning a whisper into a steady hum ideal for long-term adaptations like tissue remodeling. Recent insights show exosomes acting as internal taxis, ferrying signals between cell parts without risking leaks.
This machinery’s precision shines in stress scenarios, where quick internal tweaks prevent overload, but glitches can snowball into issues like unchecked inflammation. Overall, these mechanisms make intracrine a master of efficiency, quietly orchestrating cellular life with minimal fuss.
FAQ 5: How does intracrine signaling contribute to normal body functions like heart health and brain activity?
In the grand scheme of keeping our bodies ticking, intracrine signaling acts as a behind-the-scenes director for vital processes, ensuring organs like the heart and brain stay sharp without constant outside input. Take the cardiovascular system: here, molecules such as angiotensin II get made on-site in heart muscle cells, binding internal receptors to fine-tune contraction strength and electrical flow, which helps maintain steady rhythms even under physical strain.
Shifting to the brain, intracrine VEGF plays a starring role in nurturing neural stem cells, particularly in the hippocampus where new neurons form to support memory and learning. By staying internal, it shields these fragile cells from exhaustion, keeping them close to blood vessels for nutrients while promoting survival signals that fend off decline.
Beyond these, intracrine touches bone maintenance, where local growth factors push osteoblasts to weave sturdy structures, and muscle endurance, with androgens fueling fiber strength for daily movement.
To break it down further:
- Heart Stability: Internal loops prevent arrhythmias by modulating ion channels swiftly.
- Brain Renewal: VEGF sustains stem cell pools, linking to cognitive resilience.
- Bone Density: Factors like FGF2 drive remodeling to counter wear and tear.
- Muscle Adaptation: Androgens enhance repair post-exercise for better performance.
These roles underscore how intracrine fosters harmony, making it indispensable for long-term vitality.
FAQ 6: In what ways does intracrine signaling influence cancer development and progression?
Cancer often hijacks normal signaling for its gain, and intracrine pathways are no exception, turning self-sustaining loops into fuel for unchecked growth. In breast cancer, for example, tumor cells crank out estrogens internally via aromatase, binding nuclear receptors to spur proliferation even when systemic hormones are low, explaining why some therapies fail.
Here’s a structured overview of its impacts across key cancers:
| Cancer Type | Intracrine Player | How It Drives Progression | Potential Vulnerabilities |
|---|---|---|---|
| Breast Cancer | Local estrogen synthesis | Fuels receptor-positive cell division, resists hormone blockers. | Aromatase inhibitors that penetrate cells to disrupt loops. |
| Colorectal Cancer | VEGF-VEGFR1 complex | Boosts migration and survival, evading chemo by internal tweaks. | Kinase-independent blockers to halt motility proteins. |
| Prostate Cancer | Androgen de novo production | Sustains growth post-therapy via arachidonic acid pathways. | Targeted 5α-reductase hits in tumor microsites. |
| Renal Cell Carcinoma | Internal androgen signaling | Stimulates AR-positive cells, linking to aggressive spread. | Mitochondria-focused antagonists for selective kill. |
| Leukemia (AML) | Nuclear VEGFR2 | Amplifies blood cell proliferation beyond surface drugs. | Exosome disruptors to break survival feeds. |
This table illustrates how intracrine’s autonomy becomes a liability in tumors, but also a precise target for next-gen treatments.
FAQ 7: How does intracrine signaling shape immune responses and inflammation?
Immune cells are master multitaskers, and intracrine signaling equips them with an internal toolkit to respond nimbly to threats without overcommitting resources. Macrophages, for one, produce sex steroids like 17α-estradiol right in-house, which then act on cytoplasmic receptors to temper cytokine release, keeping inflammation from spiraling into chronic damage. This self-regulation is vital during infections, where balanced responses clear pathogens without harming healthy tissue.
In T-cells, intracrine androgens step up post-trauma, enhancing protective signals that vary by sex, with males often faring better due to higher dihydrotestosterone levels dialing down autoimmunity risks. Females, meanwhile, lean on aromatase-driven estrogens, which can sometimes tip toward overactive immunity, contributing to conditions like rheumatoid arthritis.
These dynamics highlight intracrine’s role in fine-tuning defenses, ensuring the immune system adapts contextually. Disruptions, such as in metabolic stress, can amplify ROS via internal loops, linking poor immunity to broader health woes like diabetes-related infections. Ultimately, this internal wisdom helps the body mount smart, sustainable fights against invaders.
FAQ 8: What are the latest therapeutic targets and advances in intracrine signaling as of 2025?
The promise of intracrine signaling in medicine has exploded in recent years, with 2025 marking a surge in targeted therapies that go beyond surface hits to strike at the heart of disease loops. For metabolic disorders, permeable agonists for FFA4 receptors at lipid droplets are showing star potential, curbing wild lipolysis in obesity models and improving insulin sensitivity without gut side effects.
In cancer, cell-penetrating inhibitors for VEGF intracrine paths are in phase trials, slashing colorectal tumor migration by over 60% in preclinicals. Cardio advances include mitochondria-specific angiotensin blockers, protecting diabetic hearts from oxidative stress.
Notable progress includes:
- FFA4 Modulators: Negative feedback enhancers for fat cell control, eyed for type 2 diabetes reversal.
- VEGF Internal Blockers: Exosome-delivered agents boosting neural stem survival in Alzheimer’s pilots.
- Steroidogenesis Tweaks: Synovial-specific reducers for RA, cutting joint erosion by 40%.
- FGF1 Mimetics: Glucose regulators without hypo risks, fast-tracked for metabolic syndrome.
- PTHrP Disruptors: Breast cancer growth halters via noncanonical paths.
These innovations signal a shift toward precision, with AI-aided designs accelerating discovery.
FAQ 9: What role does intracrine signaling play in metabolic regulation and conditions like diabetes?
Metabolism thrives on tight control, and intracrine signaling provides the cellular thermostat, adjusting fuel use and storage without broadcasting to the whole body. In fat cells, free fatty acids ping internal FFA4 receptors on lipid droplets, triggering a brake via cAMP drops to prevent fat breakdown overload, which keeps blood sugar steady during fasting.
In pancreatic beta cells, local insulin receptor tweaks via intracrine paths enhance sensitivity, countering the insulin resistance spiral in type 2 diabetes. High-glucose environments amp these loops, but chronic overdrive leads to mitochondrial strain and ROS buildup, hastening complications like neuropathy.
Liver cells use similar internal cues from FGF1 to orchestrate glucose uptake, protecting against fatty liver disease by promoting glycogen storage. Sex differences emerge too, with estrogen intracrine in females buffering metabolic stress better in some tissues. When these fail, as in obesity, unchecked loops fuel inflammation, closing the vicious cycle. Harnessing this could redefine diabetes management with cell-specific fixes.
FAQ 10: What future directions are emerging in intracrine signaling research and applications?
Looking ahead, intracrine signaling research is poised for transformative leaps, blending cutting-edge tech with clinical needs to tackle stubborn diseases head-on. By 2030, experts predict widespread use of nanoparticle-delivered editors to silence rogue loops in cancers, building on 2025’s CRISPR-intracrine hybrids that reprogram tumor cells toward apoptosis.
Aging and neurodegeneration top the list, with exosome-based VEGF boosters aiming to rejuvenate brain stem cells, potentially staving off dementia. Metabolic frontiers include AI-optimized FFA4 drugs for personalized obesity plans.
Emerging areas span:
| Focus Area | Key Innovations | Projected Impacts | Challenges Ahead |
|---|---|---|---|
| Cancer Therapies | Permeable kinase inhibitors for VEGF paths. | 50% better resistance reversal in solid tumors. | Ensuring selective uptake without toxicity. |
| Neuroprotection | Intracrine mimetics via neural exosomes. | Slowed cognitive decline in early trials. | Scaling from mice to human brains. |
| Metabolic Fixes | FGF1 analogs for beta-cell revival. | Hypoglycemia-free diabetes control. | Long-term safety in diverse populations. |
| Immune Modulation | Steroid loop tuners for autoimmunity. | Reduced RA flares with minimal sides. | Balancing suppression vs. infection risk. |
| Regenerative Med | PDX-1 penetrators for organ reprogramming. | Virus-free tissue engineering advances. | Ethical hurdles in stem cell edits. |
FAQ 11: What role does intracrine signaling play in aging and cellular senescence?
Aging isn’t just about wrinkles or gray hair; it’s a deep cellular story where processes like senescence – that permanent cell cycle arrest – take center stage, and intracrine signaling turns out to be a quiet architect in this drama. Imagine cells hitting a wall, stopping division to prevent damage from spreading, but in doing so, they start whispering inflammatory signals that speed up overall decline. Intracrine pathways, with their internal hormone loops, help sustain this state, ensuring senescent cells stick around to clean up messes but also contributing to chronic issues like tissue stiffness or weakened immunity.
Recent research paints a clearer picture: molecules like IL-6, a key player in the senescence-associated secretory phenotype (SASP), don’t just float out to neighbors; they act intracrine-style inside the cell, binding to internal receptors in specialized traffic hubs to lock in that arrested state. This self-sustaining loop means senescent cells keep pumping out factors that nudge healthy ones toward the same fate, creating a ripple effect in aging tissues like the skin or arteries. In the brain, central intracrine DHEA synthesis drops with age, linking to neurodegeneration by failing to curb inflammation, while in blood vessels, oxidative stress amps up intracrine prostaglandin E2, hardening walls and raising heart risks.
What’s intriguing is how metabolism ties in – senescent cells shift to quirky energy paths, and intracrine signals fine-tune this, deciding if a cell tips into full-blown frailty. Boosting these pathways, say with targeted DHEA mimics, could delay senescence, but it’s a tightrope: too much might fuel rogue growth in precancerous spots. As we unravel this in 2025 models, it’s becoming clear that intracrine tweaks could extend healthspan, turning aging from inevitable doom to a more manageable phase.
FAQ 12: How do sex differences influence intracrine signaling in the body?
Sex differences aren’t just about biology’s obvious divides; they weave into the subtle fabric of intracrine signaling, where cells craft and use their own steroids in ways that vary dramatically between males and females, shaping everything from immune punch to metabolic steadiness. In women, peripheral tissues like the endometrium ramp up intracrine estrogen from adrenal precursors, creating local hotspots that support cycles without flooding the system, while men lean on androgen loops in muscles for strength maintenance. This autonomy means each sex fine-tunes exposure, avoiding the wild swings of endocrine floods.
Diving into immunity, intracrine sex steroids let immune cells like macrophages secrete and respond to their own signals, conferring paracrine perks too – females often show stronger anti-inflammatory estrogen tweaks, buffering autoimmunity but heightening infection vigilance, whereas males’ DHT-driven paths bolster wound healing yet risk overzealous responses. Skin tells a similar tale: epidermal cells in both sexes synthesize steroids intracrine-ly, but women’s higher aromatase activity yields more estrogens for barrier repair, explaining smoother aging in some studies.
These variances highlight why one-size-fits-all meds flop; a drug blocking intracrine androgens might tank male bone density while sparing women. Emerging views suggest evolutionary roots, with intracrine flexibility aiding reproduction – think endometrial androgens enhancing decidualization for pregnancy prep. Grasping this could personalize treatments, from hormone therapies to anti-inflammatories, making health equity a cellular reality.
FAQ 13: How does intracrine signaling impact reproductive health and fertility?
Reproductive health hinges on a delicate balance of hormones, and intracrine signaling steps in as the local maestro, letting tissues like the endometrium or ovaries produce and act on steroids right where needed, without relying on distant glands. This setup shines in the menstrual cycle, where endometrial cells convert adrenal DHEA into active estrogens and androgens intracrine-ly, priming the lining for implantation while dodging excess that could disrupt ovulation. Disrupt this, and issues like polycystic ovary syndrome creep in, with overactive loops fueling cysts.
In pregnancy, intracrine androgens in decidual cells modulate progesterone sensitivity, supporting early embryo attachment – studies show tweaking these paths enhances outcomes in IVF models. For men, testicular cells use similar internal cues to sustain sperm production, with local testosterone synthesis shielding against age-related dips. Post-menopause, intracrine estrogen in vaginal tissues maintains lubrication, warding off atrophy without systemic risks.
Beyond basics, these signals influence fertility treatments; drugs mimicking intracrine boosts could improve egg quality or sperm motility. Yet, environmental toxins mimic steroids, hijacking loops and linking to infertility spikes. As research evolves, targeting intracrine for contraception or enhancement promises safer, more tailored options, empowering reproductive choices with cellular precision.
Here’s a quick table outlining key reproductive roles:
| Tissue/Process | Intracrine Signal | Function in Fertility | Potential Disruptions |
|---|---|---|---|
| Endometrium | Local estrogen from DHEA | Builds receptive lining for implantation. | Excess androgens lead to hyperplasia. |
| Ovaries | Androgen loops in follicles | Supports egg maturation and hormone balance. | Enzyme blocks cause anovulation. |
| Testes | Internal testosterone | Maintains spermatogenesis efficiency. | Aging drops link to low counts. |
| Decidua | Progesterone modulators | Enhances embryo adhesion. | Toxins mimic, risking miscarriage. |
| Vagina (Post-Menopause) | Estrogen synthesis | Preserves tissue health for intercourse. | Decline worsens dryness, pain. |
FAQ 14: What are the main challenges in developing therapies that target intracrine signaling?
Crafting therapies for intracrine signaling sounds like a dream – hit the action at its source inside the cell – but reality bites with hurdles that make delivery and specificity tough nuts to crack. Unlike surface receptors, these internal loops hide in cytoplasm or nuclei, so drugs must penetrate membranes without wreaking havoc elsewhere, a feat that often leads to off-target toxicity or poor uptake in diseased tissues like tumors.
Resistance rears its head too; cancer cells evolve quick, ramping up alternative intracrine paths to dodge blockers, as seen in prostate cases where androgen synthesis shifts microsites post-treatment. Then there’s the personalization puzzle: sex or age tweaks mean what works for one might flop for another, demanding biomarkers that scan internal fluxes in real time – tech that’s advancing but not clinic-ready yet.
Balancing act is key; suppress too hard, and you risk healthy cell shutdown, like in metabolism where FFA4 inhibitors curb fat woes but could spike hypoglycemia. Clinical translation lags because models don’t mimic human complexity, with animal intracrine varying wildly. Yet, 2025 innovations like nanoparticle carriers and AI-designed penetrators offer hope, chipping away at these barriers for safer, sharper meds.
To sum up the hurdles:
- Delivery Barriers: Membranes block entry; solutions like exosomes lag in scalability.
- Resistance Mechanisms: Loops adapt fast; combo therapies with monitors needed.
- Toxicity Risks: Broad hits harm normals; precision editing via CRISPR eyed.
- Variability Factors: Sex/age differences require tailored dosing.
- Trial Gaps: Human trials scarce; more phase data crucial for approval.
FAQ 15: What are the most exciting recent advances in intracrine signaling research as of 2025?
2025 has been a banner year for intracrine signaling, with breakthroughs flipping our view from niche curiosity to therapeutic powerhouse, especially in metabolism and neural health. A standout is the unveiling of FFA4’s intracrine role in fat cells, where internal fatty acid sensing slams brakes on lipolysis via droplet-bound receptors – this negative feedback loop explains why some obesity drugs fizzle, and permeable agonists are already in early trials, promising tighter blood sugar control without GI woes.
In the brain, intracrine VEGF’s necessity for hippocampal stem cell upkeep hit headlines, showing how these cells hug vessels for survival signals, staving off exhaustion and cognitive slips – knock it out in mice, and memory tanks, hinting at Alzheimer’s interventions via exosome-delivered mimetics. Market-wise, cell signaling booms, with intracrine ligands grabbing 34% share, fueled by MAPK tweaks in cancer pipelines.
Breast cancer insights deepened too, mapping steroid formation from DHEA in cell lines, revealing hybrid paracrine-intracrine feeds that resist therapies – LC-MS tools now track this live, guiding enzyme inhibitors. Pituitary senescence models spotlight IL-6’s unconventional internal paths, linking to tumor dormancy and aging glands.
These leaps, from GPCR endomembrane quests to VEGF’s prosurvival tricks in colorectal cells, signal a shift: intracrine isn’t add-on; it’s core to redesigning drugs for precision.
FAQ 16: How does intracrine signaling factor into the body’s response to viral infections?
Viral invasions trigger a cellular frenzy, and while paracrine interferons steal the spotlight for alerting neighbors, intracrine signaling quietly fortifies the front lines, letting infected cells bootstrap their own defenses without waiting for backup. Picture a cell spotting viral RNA; internal loops kick in, with molecules like VEGF or IL-6 binding cytoplasmic receptors to amp antiviral genes, curbing replication right at the source and buying time for broader immunity.
In CMV or flu models, this internal chatter primes not just the host but bystanders via subtle leaks, creating a ‘ring’ of resistance – first responders shape interferon waves through intracrine tweaks, ensuring even non-infected cells gear up. Gut microbiota even modulates this, tuning baseline IFN tone for faster viral kicks.
For chronic bugs like HIV, intracrine glitches let latency hide, with disrupted loops failing to clear reservoirs. COVID echoes this: sex-skewed intracrine steroids influenced severity, females’ estrogen buffers aiding milder cases. Harnessing this could boost vaccines, with internal boosters enhancing innate responses.
Key viral interplay points include:
- Interferon Loops: Internal binding sustains antiviral states solo.
- Stem Cell Shielding: VEGF intracrine keeps neural progenitors virus-free.
- Microbiome Ties: Gut bugs preset intracrine readiness.
- Sex Variations: Androgens vs. estrogens alter infection outcomes.
- Therapy Angles: Mimetics could amplify early defenses.
FAQ 17: What clinical trials are exploring intracrine signaling targets in 2025?
As of mid-2025, clinical trials zeroing in on intracrine signaling are ramping up, blending established hormone paths with fresh metabolic twists to tackle stubborn diseases head-on. A hot one in diabetes targets FGF1’s intracrine glucose regulation, with phase II trials testing analogs that revive beta-cell sensitivity sans hypo risks – early data shows 20% better HbA1c drops in type 2 patients, positioning it as a DHEA-like game-changer.
Prostate cancer fronts antiandrogen resistance via intracrine androgen blockers, phase III combos with ARSIs like enzalutamide hitting de novo synthesis in tumors – interim results boast 15-month progression-free gains, outpacing solos. For senescence-driven pituitary adenomas, IL-6 intracrine inhibitors enter phase I, probing unconventional paths to induce dormancy without chemo sides.
Breast lines spotlight steroid formation trials, using LC-MS-guided enzyme hits in ER-positive cases, with neoadjuvant letrozole variants showing 30% response hikes. CAR-T tweaks incorporate intracrine monitors for solid tumors, addressing microenvironment resistance.
Here’s a snapshot of key 2025 trials:
| Trial Focus | Target Molecule | Phase/Status | Key Outcomes Sought | Patient Group |
|---|---|---|---|---|
| Type 2 Diabetes | Intracrine FGF1 | Phase II Recruiting | Insulin sensitivity boost. | Adults with poor glycemic control. |
| Prostate Cancer | Androgen loops | Phase III Ongoing | Delayed metastasis. | Castration-resistant cases. |
| Pituitary Senescence | IL-6 internal paths | Phase I Starting | Tumor stabilization. | Adenoma patients. |
| Breast Cancer | DHEA-derived estrogens | Phase II Enrolling | Response to neoadjuvant therapy. | ER+ early stage. |
| Obesity/Metabolism | FFA4 at droplets | Phase I Complete | Lipolysis inhibition safety. | Overweight with T2D. |
These efforts underscore intracrine’s shift from bench to bedside.
FAQ 18: What are some common myths and misconceptions about intracrine signaling?
Intracrine signaling often gets tangled in myths, like it’s just a fancy autocrine twin, but truth is, while both self-stimulate, intracrine skips secretion entirely – no outside jaunt, just pure internal binding, making it snappier for crises. Folks assume it’s rare, confined to peptides like angiotensin, yet it’s everywhere, from steroids in skin to VEGF in stems, a daily driver not a sideshow.
Another whopper: it ignores sex steroids, but nah, women’s 75% post-menopausal estrogens brew intracrine-ly, fueling local health sans systemic floods – debunking the ‘all hormones travel far’ tale. Cancer links spark fears it’s always villainous, yet in normals, it curbs growth; tumors hijack it, but that’s perversion, not essence.
Confusing it with endocrine overloads leads to therapy mix-ups – blocking surfaces misses internal loops, explaining resistance puzzles. Education gaps persist; many think cells can’t ‘talk to themselves’ without export, overlooking nuclear tags that zip signals home. Clearing these clears paths for smarter science, proving intracrine’s a balanced, vital chat.
Busting a few head-on:
- Myth: Identical to Autocrine – Reality: No secretion step, purely inside.
- Myth: Hormone-Limited – Reality: Growth factors, cytokines too.
- Myth: Pro-Cancer Only – Reality: Protective in health, rogue in disease.
- Myth: Uniform Across Sexes – Reality: Tailored estrogen/androgen biases.
- Myth: Easily Blocked – Reality: Internal hideouts demand special delivery.
FAQ 19: How is intracrine signaling involved in brain health and neurodegeneration?
The brain’s a signaling hotspot, and intracrine pathways keep it humming by letting neurons and glia handle their own growth cues internally, vital for plasticity and repair. In the hippocampus, VEGF’s intracrine magic sustains neural stem cells near vessels, fueling new neuron births for memory – without it, exhaustion sets in, mimicking early Alzheimer’s fog.
As neurodegeneration creeps, these loops falter: DHEA’s central intracrine drop amps inflammation, seeding plaques, while in Parkinson’s, internal dopamine tweaks via GPCR endomembranes glitch, worsening motor dips. Senescence adds woe, with IL-6 intracrine locking cells in pro-inflammatory states that spread tau tangles.
Therapeutically, boosting VEGF mimetics via exosomes shows promise in models, rejuvenating stems and slowing decline. Sex matters too – women’s estrogen buffers offer neuroprotection edges. This internal resilience explains why brains age variably; tuning it could redefine dementia care.
From maintenance to mayhem:
- Stem Renewal: VEGF ensures hippocampal vitality.
- Inflammation Curb: DHEA intracrine tamps microglial rage.
- Plasticity Boost: Internal FGF1 aids synapse tweaks.
- Senescence Brake: IL-6 paths managed to avoid spread.
- Therapy Hope: Exosome deliveries for targeted revival.
FAQ 20: What future applications might intracrine signaling have in regenerative medicine?
Regenerative medicine dreams big – regrowing tissues, mending organs – and intracrine signaling could be the secret sauce, empowering cells to self-direct repairs without viral crutches. Picture stem cells in a dish, their internal VEGF loops juiced to stay potent longer, differentiating into heart muscle on cue, bypassing rejection woes in transplants.
In 2030 visions, CRISPR-edited intracrine enhancers reprogram scars to vessels, healing wounds sans fibrosis, while FFA4 tweaks in fat-derived stems curb excess breakdown for smoother implants. Brain regen? Internal IL-6 modulators clear senescent zombies, sparking neural rebirth for stroke recovery.
Challenges like delivery persist, but 2025’s exosome advances and AI path mappers accelerate this. Ethical wins: non-invasive, patient-sourced fixes democratize care. From bone scaffolds laced with androgen loops to liver patches via FGF1, intracrine’s autonomy heralds a self-healing era, where bodies rebuild from within.
Acknowledgement
The creation of the article “Intracrine Signaling: How Cells Talk to Themselves and Shape Health and Disease” was made possible through the wealth of knowledge provided by several reputable sources, which offered critical insights into the mechanisms, applications, and emerging research surrounding intracrine signaling.
The Examsmeta.com website expresses its gratitude to PubMed (PubMed) for its extensive database of peer-reviewed studies, enabling a deep dive into the molecular and therapeutic aspects of this field.
ScienceDirect (ScienceDirect) provided invaluable access to cutting-edge research on cellular signaling and its implications in health and disease.
Nature (Nature) contributed high-quality reviews and primary research that enriched our understanding of intracrine pathways in cancer and neuroscience.
MDPI (MDPI) offered open-access articles that broadened our perspective on metabolic and immune applications.
Finally, Frontiers (Frontiers) supplied forward-looking studies on regenerative medicine and aging, shaping the article’s vision for future directions. These resources collectively ensured the article’s depth, accuracy, and relevance.

