Synaptic signaling forms the backbone of how our nervous system operates, allowing billions of neurons to exchange information rapidly and precisely. This process is essential for everything from simple reflexes to complex thoughts and emotions. Synaptic signaling involves neurons passing signals across tiny gaps called synapses, either through chemical messengers or direct electrical connections. These interactions enable the brain and body to respond to stimuli, learn new skills, and maintain bodily functions. While most people might not think about it daily, disruptions in this signaling can lead to various health issues, highlighting its importance in overall well-being.
The concept of synapses was first introduced over a century ago, but our understanding has grown tremendously with advances in neuroscience. Neurons, the specialized cells of the nervous system, don’t touch each other directly; instead, they communicate at these junctions. This communication can excite or inhibit the receiving cell, shaping how signals propagate through networks. Whether it’s the quick snap of a muscle in response to touch or the intricate processing behind decision-making, synaptic signaling is always at play.
In this article, we’ll dive deep into the mechanisms, types, and real-world implications of this fascinating process.
Table of Contents
The Basics of Synapses: Junctions Where Neurons Meet
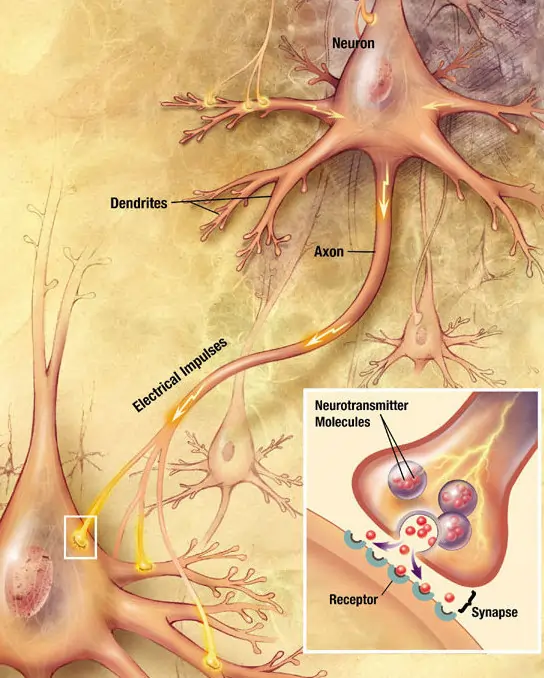
A synapse is essentially a specialized connection between two neurons or between a neuron and a target cell like a muscle or gland. It’s where the magic of neural communication happens. The neuron sending the signal is known as the presynaptic neuron, while the one receiving it is the postsynaptic neuron. This setup ensures that information flows in a directed manner, from one cell to another.
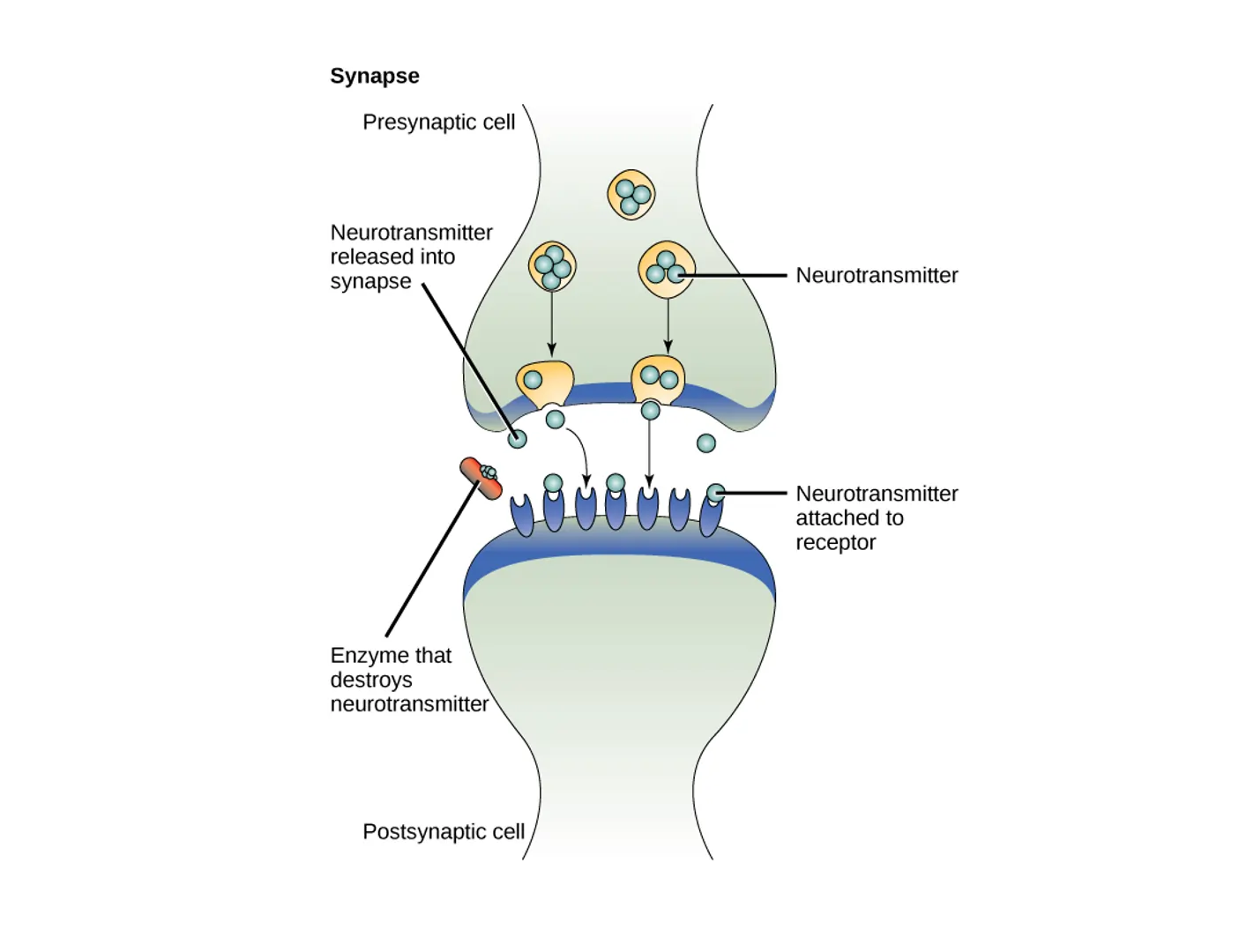
Synapses come in two main flavors: chemical and electrical. Chemical synapses are far more common in the human nervous system and involve the release of neurotransmitters, which are chemical substances that carry the signal across a small gap called the synaptic cleft. Electrical synapses, on the other hand, allow direct ion flow between cells through structures called gap junctions, making them faster but less flexible.
The structure of a synapse is intricate. In chemical synapses, the presynaptic end features an axon terminal packed with vesicles containing neurotransmitters. The postsynaptic side has receptors ready to bind these chemicals. This design allows for precise control over signal strength and type. For instance, some synapses amplify signals, while others dampen them, contributing to the brain’s computational power.
Beyond structure, synapses are dynamic. They can strengthen or weaken over time through processes like long-term potentiation or depression, which are key to learning and memory. This plasticity means synapses aren’t static; they adapt based on experience, helping us form habits or recall information.
To visualize this, imagine a busy highway intersection where cars (signals) must cross safely. The presynaptic neuron is like the incoming road, releasing messengers like delivery trucks across the cleft to influence the outgoing traffic on the postsynaptic side.
Chemical Synapses: The Predominant Form of Neural Communication
Chemical synapses dominate the nervous system because they offer versatility in signaling. When an action potential—a brief electrical spike—reaches the axon terminal of the presynaptic neuron, it triggers a cascade of events. Voltage-gated calcium channels open, allowing calcium ions to flood in from outside the cell, where their concentration is higher.
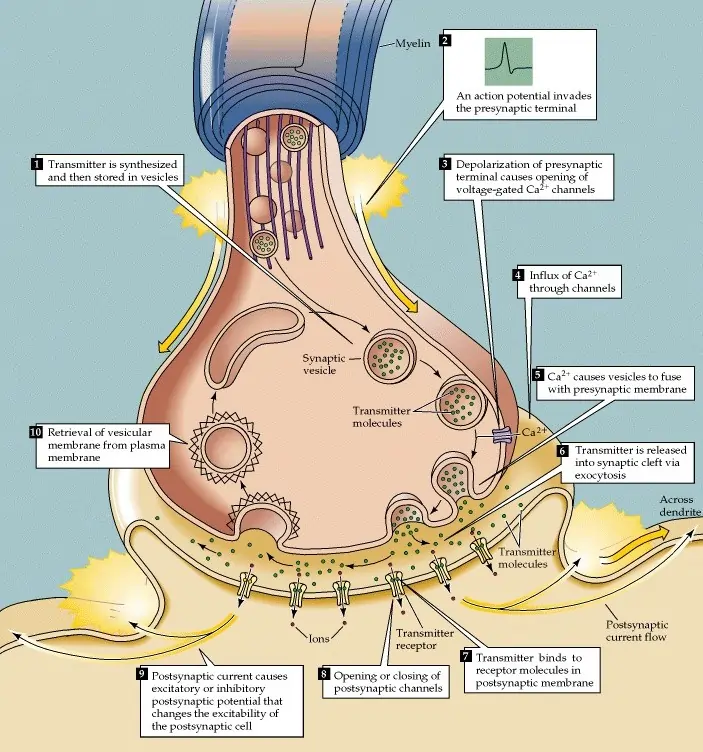
This influx of calcium prompts vesicles filled with neurotransmitters to fuse with the cell membrane, releasing their contents into the synaptic cleft. The neurotransmitters then diffuse across this narrow space—typically about 20-40 nanometers wide—and bind to specific receptors on the postsynaptic neuron.
Binding can lead to various effects. For excitatory synapses, it often opens ion channels that let positive ions like sodium enter, depolarizing the membrane and making an action potential more likely. Inhibitory synapses might allow chloride ions in, hyperpolarizing the cell and reducing firing chances. This balance is crucial for proper neural function.
After signaling, the process must reset. Neurotransmitters are removed from the cleft through enzymatic breakdown, reuptake into the presynaptic neuron, diffusion away, or uptake by nearby glial cells. This clearance prevents constant stimulation and allows for precise timing in communication.
Chemical synapses can modulate signals in ways electrical ones cannot. For example, a single presynaptic release might trigger a small response, but repeated firings can build up, leading to stronger effects. This ability to amplify or transform signals makes them ideal for complex processing in the brain.
Consider the neuromuscular junction, where a motor neuron signals a muscle fiber. Here, the neurotransmitter acetylcholine binds to receptors, causing muscle contraction. This is a classic example of chemical synaptic signaling in action, essential for movement.
Types of Neurotransmitters: Messengers with Diverse Roles
Neurotransmitters are the stars of chemical synapses, each with unique functions that influence mood, movement, and more. They can be classified into several categories based on their chemical structure and effects.
Small-molecule neurotransmitters act quickly and include amino acids like glutamate and GABA. Glutamate is the primary excitatory neurotransmitter in the brain, promoting neuron firing and involved in learning. GABA, conversely, is inhibitory, calming neural activity and helping prevent overstimulation, which is vital for sleep and anxiety control.
Monoamines, another group, include dopamine, serotonin, and norepinephrine. Dopamine plays a key role in reward and motivation, with imbalances linked to conditions like Parkinson’s disease. Serotonin regulates mood, appetite, and sleep, often targeted by antidepressants. Norepinephrine heightens alertness and is involved in the fight-or-flight response.
Peptides like endorphins act as natural painkillers, modulating pain and stress. Gasotransmitters such as nitric oxide are unique, diffusing freely and influencing blood flow and synaptic plasticity.
Here’s a comprehensive table outlining major neurotransmitters, their types, primary functions, and examples of roles in the body:
| Neurotransmitter | Type | Primary Function | Key Roles in the Body | Examples of Effects |
|---|---|---|---|---|
| Glutamate | Amino Acid | Excitatory | Promotes neuron firing, essential for synaptic plasticity and learning | Involved in memory formation; excess can lead to excitotoxicity in strokes |
| GABA (Gamma-Aminobutyric Acid) | Amino Acid | Inhibitory | Reduces neural excitability, promotes calm | Aids in anxiety reduction; targeted by anti-anxiety medications like benzodiazepines |
| Glycine | Amino Acid | Inhibitory (mainly in spinal cord) | Inhibits motor neurons, helps with muscle tone | Plays a role in reflex arcs; imbalances can cause spasticity |
| Acetylcholine | Small Molecule | Excitatory or Inhibitory (depending on receptor) | Muscle contraction, autonomic functions | At neuromuscular junctions for movement; in brain for attention and memory |
| Dopamine | Monoamine | Modulatory | Reward, motivation, motor control | Drives pleasure from food or achievements; low levels in Parkinson’s lead to tremors |
| Serotonin | Monoamine | Modulatory | Mood regulation, sleep, appetite | Influences happiness; deficiencies linked to depression |
| Norepinephrine | Monoamine | Excitatory | Alertness, stress response | Boosts heart rate during danger; involved in ADHD treatments |
| Epinephrine (Adrenaline) | Monoamine | Excitatory | Fight-or-flight response | Increases energy during emergencies; not primarily a brain neurotransmitter |
| Endorphins | Peptide | Inhibitory | Pain relief, euphoria | Released during exercise; natural opioids reducing discomfort |
| Substance P | Peptide | Excitatory | Pain transmission | Signals pain in spinal cord; targeted in some pain therapies |
| Nitric Oxide | Gasotransmitter | Modulatory | Vasodilation, synaptic plasticity | Improves blood flow; aids in long-term memory |
| Histamine | Monoamine | Excitatory | Wakefulness, allergic responses | Promotes alertness; antihistamines cause drowsiness |
This table highlights the diversity of neurotransmitters, showing how they orchestrate a symphony of bodily functions. Each one can bind to multiple receptor types, adding layers of complexity to signaling.
For instance, in the reward pathway, dopamine release reinforces behaviors like eating or socializing, creating a sense of pleasure that encourages repetition.
Electrical Synapses: Fast and Direct Connections
While chemical synapses are versatile, electrical synapses prioritize speed. They connect neurons via gap junctions, protein channels that allow ions and small molecules to pass directly between cells’ cytoplasms. This direct link means signals transmit almost instantaneously, without the delay of chemical release and binding.
Electrical synapses are bidirectional, allowing signals to flow both ways, unlike the one-way nature of most chemical synapses. However, they can’t amplify signals or switch from excitatory to inhibitory easily, limiting their use to specific scenarios.
In humans, electrical synapses are found in regions needing synchronized activity, such as the retina for visual processing or cardiac muscle for coordinated heartbeats. In invertebrates, they’re more common, like in squid escape responses where rapid signaling is life-saving.
A key difference is size: electrical synaptic clefts are narrower, about 3-5 nanometers, compared to chemical ones. This closeness enables faster transmission, ideal for reflexes.
Here’s a detailed comparison table between chemical and electrical synapses:
| Feature | Chemical Synapses | Electrical Synapses |
|---|---|---|
| Transmission Mechanism | Release of neurotransmitters across synaptic cleft | Direct ion flow through gap junctions |
| Speed | Slower (milliseconds due to diffusion and binding) | Faster (nearly instantaneous) |
| Directionality | Unidirectional (presynaptic to postsynaptic) | Bidirectional |
| Flexibility | High; can amplify, inhibit, or modulate signals | Low; fixed transmission without modulation |
| Prevalence | Most common in vertebrate nervous systems | Less common; found in specific areas like retina or heart |
| Synaptic Cleft Width | 20-40 nm | 3-5 nm |
| Energy Requirement | Requires ATP for vesicle fusion and reuptake | Minimal; passive ion flow |
| Examples | Neuromuscular junction (acetylcholine), brain excitatory synapses (glutamate) | Squid giant axon for escape, mammalian retinal cells for synchronization |
| Advantages | Allows complex integration and plasticity | Enables rapid, synchronized activity |
| Disadvantages | Prone to fatigue from neurotransmitter depletion | Cannot transform signal type |
This table underscores why the nervous system uses both types strategically. For quick, invariant responses, electrical wins; for nuanced processing, chemical prevails.
An example is in the crayfish tail-flip escape, where electrical synapses ensure all motor neurons fire together for a swift getaway.
Synaptic Integration: How Neurons Decide to Fire
Neurons don’t act in isolation; they integrate multiple inputs to decide whether to send a signal. This process, called synaptic integration, occurs mainly in the dendrites and cell body of the postsynaptic neuron.
Inputs can be excitatory postsynaptic potentials (EPSPs) that depolarize or inhibitory postsynaptic potentials (IPSPs) that hyperpolarize. The neuron sums these spatially (from different synapses) and temporally (over time).
If the net depolarization reaches a threshold at the axon hillock, an action potential fires. This all-or-none response means integration is the gatekeeper.
Factors like synapse location matter: distal inputs weaken before reaching the hillock, while proximal ones have more impact.
In cortical neurons, dendrites can perform local computations, enhancing processing power.
For example, in decision-making, thousands of inputs from sensory areas integrate to trigger a motor response, like catching a ball.
Bullet points on types of synaptic integration:
- Spatial Summation: Adding EPSPs from multiple synapses at once to reach threshold.
- Temporal Summation: Rapid successive EPSPs from one synapse building up.
- Shunting Inhibition: IPSPs near the soma blocking excitation effectively.
- Two-Stage Integration: Dendritic branches process locally before soma summation.
This integration allows neurons to act as sophisticated computers, filtering noise and prioritizing signals.
Real-World Examples of Synaptic Signaling in the Human Body
Synaptic signaling isn’t abstract; it’s behind everyday actions. At the neuromuscular junction, motor neurons release acetylcholine to contract muscles, enabling walking or typing.
In the brain, glutamate synapses in the hippocampus support memory formation during learning.
The autonomic nervous system uses synapses for involuntary functions: norepinephrine synapses speed up the heart during stress.
In sensory processing, retinal electrical synapses synchronize cells for better light detection.
Pain pathways involve substance P synapses transmitting nociceptive signals to the brain.
Reflex arcs, like the knee-jerk, showcase simple integration: stretch sensors synapse directly with motor neurons.
In the reward system, dopamine synapses in the nucleus accumbens reinforce addictive behaviors.
These examples illustrate how synaptic signaling coordinates the body’s responses seamlessly.
Disorders Linked to Faulty Synaptic Signaling
When synaptic signaling goes awry, it can lead to numerous disorders. Many neurodevelopmental conditions stem from synaptic gene mutations, affecting communication.
Autism spectrum disorders often involve disrupted synaptic plasticity, impairing social cues processing.
Schizophrenia may arise from abnormal dopamine signaling and synaptic pruning issues during development.
In Alzheimer’s disease, amyloid plaques disrupt synapses, leading to memory loss.
Parkinson’s features dopamine neuron loss, weakening motor control synapses.
Epilepsy results from imbalanced excitation-inhibition, causing seizures.
Depression links to low serotonin synaptic activity, affecting mood.
Genetic disorders like fragile X syndrome impair synaptic maturation.
Addiction hijacks reward synapses, altering motivation.
Here’s an extensive table of disorders, their synaptic links, symptoms, and treatments:
| Disorder | Synaptic Dysfunction | Key Symptoms | Common Treatments | Affected Neurotransmitters |
|---|---|---|---|---|
| Autism Spectrum Disorders | Impaired synaptic plasticity and connectivity | Social difficulties, repetitive behaviors | Behavioral therapy, medications for symptoms | Glutamate, GABA |
| Schizophrenia | Abnormal dopamine signaling, reduced synaptic density | Hallucinations, delusions, cognitive deficits | Antipsychotics | Dopamine, glutamate |
| Alzheimer’s Disease | Synaptic loss due to plaques and tangles | Memory impairment, confusion | Cholinesterase inhibitors | Acetylcholine, glutamate |
| Parkinson’s Disease | Degeneration of dopamine-producing synapses | Tremors, rigidity, slow movement | Levodopa, deep brain stimulation | Dopamine |
| Epilepsy | Excitatory-inhibitory imbalance at synapses | Seizures, loss of consciousness | Anticonvulsants | GABA, glutamate |
| Major Depressive Disorder | Reduced serotonin and norepinephrine synaptic activity | Persistent sadness, low energy | SSRIs, therapy | Serotonin, norepinephrine |
| Attention Deficit Hyperactivity Disorder (ADHD) | Dysregulated dopamine and norepinephrine synapses | Inattention, hyperactivity | Stimulants like methylphenidate | Dopamine, norepinephrine |
| Bipolar Disorder | Fluctuating synaptic modulation | Mood swings, mania, depression | Mood stabilizers | Serotonin, dopamine |
| Fragile X Syndrome | Defective synaptic maturation | Intellectual disability, anxiety | Supportive therapies | Glutamate |
| Addiction | Overstimulation of reward synapses | Compulsive behavior, withdrawal | Counseling, medications like methadone | Dopamine |
| Myasthenia Gravis | Autoimmune attack on acetylcholine receptors | Muscle weakness, fatigue | Acetylcholinesterase inhibitors | Acetylcholine |
| Huntington’s Disease | Mutant protein disrupting synaptic function | Chorea, cognitive decline | Tetrabenazine for symptoms | GABA, glutamate |
This table shows the broad impact of synaptic issues, emphasizing the need for targeted therapies.
Research continues to uncover how fixing synaptic defects could treat these conditions, offering hope for future interventions.
The Evolutionary Perspective on Synaptic Signaling
Synapses have evolved to meet the demands of increasingly complex organisms. In simple animals like jellyfish, basic electrical-like connections suffice for movement. As nervous systems advanced, chemical synapses allowed for finer control, enabling behaviors like hunting or social interaction.
In vertebrates, the mix of both types optimizes speed and adaptability. This evolution underscores why humans can perform intricate tasks, from playing instruments to solving puzzles.
Future Directions in Synaptic Research
Ongoing studies explore synaptic roles in aging and regeneration. Techniques like optogenetics allow precise control of synapses, revealing their mechanics.
Understanding these could lead to breakthroughs in treating synaptic-related disorders, enhancing cognitive function, or even interfacing brains with computers.
In summary, synaptic signaling is a cornerstone of life, blending chemistry and electricity to create the tapestry of human experience. By appreciating its details, we gain insight into our own minds and bodies.
Frequently Asked Questions
FAQ 1: What Is Synaptic Signaling and Why Is It Important?
Synaptic signaling represents the fundamental way neurons in our nervous system communicate with each other and with other cells, like those in muscles or glands. At its essence, this process involves the transfer of information across tiny junctions known as synapses, where an electrical or chemical signal from one neuron influences the activity of another. This communication can either excite the receiving cell, making it more likely to fire its own signal, or inhibit it, calming down potential activity. Without synaptic signaling, our brains couldn’t process thoughts, coordinate movements, or respond to the world around us. It’s the intricate network that turns individual neuron firings into coherent actions and perceptions.
The importance of synaptic signaling extends far beyond basic biology. It underpins learning, memory, and even emotional responses. For instance, when you learn a new skill, such as riding a bike, repeated synaptic signals strengthen connections between neurons, making the pathway more efficient over time. Disruptions in this signaling can lead to serious health issues, including neurological disorders. Research shows that synaptic dysfunction plays a role in conditions like depression, where imbalances in signaling chemicals affect mood regulation. Moreover, synaptic signaling is energy-efficient, allowing the brain to handle vast amounts of data without overheating or exhausting resources.
In evolutionary terms, synaptic signaling has been a game-changer for complex life forms. It enables rapid adaptations to environments, from a squid’s quick escape response to human problem-solving. As our understanding grows, scientists are exploring how enhancing synaptic signaling could improve cognitive therapies or even combat aging-related decline in brain function.
FAQ 2: How Do Chemical Synapses Function in the Nervous System?
Chemical synapses are the most prevalent type in the human body, relying on neurotransmitters to bridge the gap between neurons. The process begins when an action potential, a swift electrical impulse, travels down the axon of the presynaptic neuron. Upon reaching the axon terminal, this impulse triggers voltage-gated calcium channels to open, allowing calcium ions to enter the cell rapidly due to higher concentrations outside.
This calcium influx causes vesicles containing neurotransmitters to fuse with the presynaptic membrane, releasing these chemical messengers into the synaptic cleft—a narrow space separating the two neurons. The neurotransmitters then diffuse across this cleft and bind to specific receptors on the postsynaptic neuron, inducing changes like the opening of ion channels. These alterations modify the postsynaptic cell’s membrane potential, potentially leading to its own action potential if the signal is strong enough.
To prevent ongoing stimulation, the signaling concludes through various mechanisms: enzymes break down the neurotransmitters, they are reabsorbed by the presynaptic neuron for reuse, or they simply diffuse away. Glial cells also assist in clearing the cleft. This cycle ensures precise and timely communication. Advanced studies reveal that chemical synapses can adapt their strength, a feature crucial for synaptic plasticity, which supports long-term changes in neural circuits.
The versatility of chemical synapses allows them to handle complex tasks, such as modulating pain signals or regulating heart rate. In contrast to faster but less adaptable electrical synapses, chemical ones provide the flexibility needed for nuanced brain functions, making them indispensable for higher cognition.
FAQ 3: What Are the Key Differences Between Chemical and Electrical Synapses?
Chemical and electrical synapses serve as vital communication points in the nervous system, but they differ significantly in structure, function, and application. Understanding these differences helps explain why the body uses both types strategically for various tasks.
| Aspect | Chemical Synapses | Electrical Synapses |
|---|---|---|
| Transmission Method | Involves release of neurotransmitters across a synaptic cleft | Direct flow of ions through gap junctions connecting cell cytoplasms |
| Speed of Signal | Slower, typically in milliseconds due to diffusion and receptor binding | Extremely fast, almost instantaneous as there’s no chemical intermediary |
| Direction of Signal | Unidirectional, from presynaptic to postsynaptic neuron | Bidirectional, allowing signals to pass in both directions |
| Flexibility and Modulation | High; can amplify, inhibit, or alter signals based on neurotransmitter type and receptor response | Low; signals pass without modification or amplification |
| Synaptic Cleft Size | Wider, around 20-40 nanometers | Narrower, about 3-5 nanometers, enabling direct contact |
| Energy Requirements | Higher, needing ATP for vesicle fusion, release, and reuptake processes | Lower, relying on passive ion diffusion without active transport |
| Prevalence in the Body | Most common, especially in the brain and spinal cord for complex processing | Less common, found in areas needing synchronization like the heart or retina |
| Role in Plasticity | Supports long-term changes like learning through adjustable strength | Limited plasticity; more fixed in function |
| Examples in Animals | Neuromuscular junctions using acetylcholine for muscle contraction; brain synapses with glutamate for excitation | Squid escape responses for rapid coordination; mammalian cardiac muscle for heartbeat synchronization |
| Advantages | Allows for diverse responses and integration of multiple inputs | Ensures quick, reliable transmission for invariant actions |
| Disadvantages | Can fatigue if neurotransmitters deplete; susceptible to toxins | Cannot easily switch between excitatory and inhibitory effects |
| Evolutionary Notes | Evolved for adaptability in complex nervous systems | Retained from simpler organisms for speed in critical reflexes |
These distinctions highlight how chemical synapses excel in nuanced, adaptable communication, while electrical ones prioritize speed and synchrony, contributing to the overall efficiency of neural networks.
FAQ 4: What Are the Major Neurotransmitters and Their Primary Functions?
Neurotransmitters are essential chemical messengers that facilitate communication across synapses. They influence a wide array of bodily and mental functions, from movement to mood. Below is a detailed table outlining key neurotransmitters, their classifications, main roles, and associated effects or conditions.
| Neurotransmitter | Classification | Primary Function | Key Roles and Effects | Related Conditions or Notes |
|---|---|---|---|---|
| Glutamate | Amino Acid | Excitatory | Main driver of neuron firing; crucial for learning, memory, and synaptic plasticity | Excess linked to excitotoxicity in strokes; most abundant in the brain |
| GABA (Gamma-Aminobutyric Acid) | Amino Acid | Inhibitory | Reduces neural excitability; promotes relaxation and sleep | Imbalances contribute to anxiety disorders; targeted by sedatives |
| Acetylcholine | Small Molecule | Excitatory or Inhibitory | Controls muscle contractions; involved in attention, learning, and autonomic functions | Deficits in Alzheimer’s disease; used at neuromuscular junctions |
| Dopamine | Monoamine | Modulatory | Regulates reward, motivation, and motor control | Low levels in Parkinson’s; high in addiction pathways |
| Serotonin | Monoamine | Modulatory | Influences mood, appetite, sleep, and pain perception | Deficiencies associated with depression; boosted by SSRIs |
| Norepinephrine | Monoamine | Excitatory | Enhances alertness, focus, and stress responses | Involved in fight-or-flight; treatments for ADHD |
| Endorphins | Peptide | Inhibitory | Acts as natural painkillers; induces euphoria | Released during exercise or stress; similar to opioids |
| Nitric Oxide | Gasotransmitter | Modulatory | Regulates blood flow and synaptic plasticity | Aids in memory formation; vasodilator effects |
| Histamine | Monoamine | Excitatory | Promotes wakefulness; involved in allergic responses | Antihistamines cause drowsiness; role in immune-brain crosstalk |
| Glycine | Amino Acid | Inhibitory | Inhibits motor neurons; helps with muscle tone control | Imbalances can lead to spasticity in spinal cord issues |
| Substance P | Peptide | Excitatory | Transmits pain signals; involved in inflammation | Targeted in chronic pain therapies |
| Epinephrine (Adrenaline) | Monoamine | Excitatory | Amplifies fight-or-flight; increases heart rate and energy | Primarily peripheral but influences brain arousal |
This table captures the diversity of neurotransmitters, showing how they orchestrate everything from basic reflexes to complex emotions, with ongoing research revealing their interconnected roles in health and disease.
FAQ 5: How Does Synaptic Integration Occur in Neurons?
Synaptic integration is the sophisticated process where a neuron combines multiple incoming signals to decide whether to fire an action potential. This happens primarily in the dendrites and cell body, where excitatory and inhibitory inputs from thousands of synapses are summed up spatially and temporally. If the net effect depolarizes the membrane enough to reach a threshold at the axon hillock, the neuron activates, propagating the signal onward. This integration acts as a computational filter, allowing neurons to process information like tiny decision-makers.
Key aspects of synaptic integration include:
- Spatial summation, where signals from different synapses add up simultaneously to push the neuron toward firing.
- Temporal summation, involving rapid successive inputs from the same synapse building upon each other.
- Inhibitory influences that can shunt or cancel out excitation, maintaining balance to prevent overactivity.
- Dendritic computations, where branches perform local processing before signals reach the soma, enhancing complexity.
- Voltage-gated channels in dendrites that can amplify or attenuate signals based on location and timing.
This mechanism ensures neurons respond appropriately to diverse stimuli, from sensory inputs to internal states, forming the basis for reflexes, thoughts, and behaviors.
FAQ 6: What Role Does Synaptic Plasticity Play in Learning and Memory?
Synaptic plasticity refers to the ability of synapses to strengthen or weaken over time in response to activity, forming the neural basis for learning and memory. Processes like long-term potentiation, or LTP, enhance synaptic efficiency when neurons fire together frequently, making future communication easier. This “cells that fire together wire together” principle allows experiences to reshape brain circuits, encoding new information durably.
Conversely, long-term depression, or LTD, weakens underused connections, refining networks by pruning irrelevant pathways. Together, LTP and LTD maintain a dynamic balance, preventing overload while adapting to new knowledge. For example, in the hippocampus, repeated stimulation during learning induces LTP, solidifying memories of events or skills. Synaptic plasticity isn’t limited to youth; it persists throughout life, supporting lifelong learning and recovery from injuries.
Factors like stress, sleep, and nutrition influence plasticity. Adequate sleep consolidates memories by replaying synaptic patterns, while chronic stress can impair LTP, affecting cognitive health. Emerging therapies target plasticity to treat memory disorders, highlighting its central role in mental agility.
FAQ 7: What Are Common Neurological Disorders Linked to Synaptic Dysfunction?
Synaptic dysfunction underlies many neurological and psychiatric conditions, where faulty signaling disrupts normal brain function. The following table details prominent disorders, their synaptic connections, symptoms, and potential treatments, drawing from extensive research.
| Disorder | Synaptic Link | Main Symptoms | Treatment Approaches | Notable Insights |
|---|---|---|---|---|
| Alzheimer’s Disease | Loss of synapses due to plaques; reduced acetylcholine signaling | Memory loss, confusion, cognitive decline | Cholinesterase inhibitors; lifestyle interventions | Early synaptic changes precede neuron death |
| Parkinson’s Disease | Degeneration of dopamine synapses in motor areas | Tremors, stiffness, slowed movements | Dopamine precursors like levodopa | Involves synaptic pruning errors |
| Schizophrenia | Abnormal dopamine and glutamate synapses; reduced density | Hallucinations, delusions, thought disorders | Antipsychotics targeting dopamine | Linked to developmental synaptic issues |
| Autism Spectrum Disorders | Impaired plasticity and connectivity | Social challenges, repetitive behaviors | Behavioral therapies; symptom-specific meds | Genetic mutations affect synapse formation |
| Epilepsy | Imbalance in excitatory-inhibitory synapses | Seizures, altered consciousness | Anticonvulsants enhancing GABA | Synaptic overexcitation triggers episodes |
| Major Depressive Disorder | Diminished serotonin and norepinephrine activity | Persistent low mood, fatigue | Antidepressants like SSRIs | Plasticity deficits hinder recovery |
| Attention Deficit Hyperactivity Disorder (ADHD) | Dysregulated dopamine synapses | Inattention, impulsivity | Stimulants boosting dopamine | Synaptic modulation improves focus |
| Huntington’s Disease | Protein aggregates disrupt synaptic function | Involuntary movements, cognitive impairment | Symptom management with tetrabenazine | Progressive synaptic loss in striatum |
| Amyotrophic Lateral Sclerosis (ALS) | Synaptic vulnerability in motor neurons | Muscle weakness, paralysis | Riluzole for glutamate regulation | Early synaptic changes in disease progression |
| Fragile X Syndrome | Defective glutamate synapse maturation | Intellectual disability, behavioral issues | Supportive therapies | Leading cause of inherited synaptic disorder |
| Bipolar Disorder | Fluctuating synaptic modulation | Extreme mood swings | Mood stabilizers like lithium | Involves ion channel synapse effects |
This overview illustrates how targeting synaptic mechanisms could revolutionize treatments for these widespread conditions.
FAQ 8: How Have Synapses Evolved in Different Animals?
The evolution of synapses marks a pivotal advancement in animal nervous systems, transitioning from simple diffusion-based signaling in early organisms to sophisticated networks in complex creatures. In primitive animals like sponges, precursors to synapses existed as basic chemical releases without true junctions. As multicellular life advanced, electrical synapses emerged in cnidarians, such as jellyfish, enabling rapid ion flow for basic movements and responses.
Chemical synapses appeared later, offering greater flexibility, and became dominant in bilaterians like worms and insects. This shift allowed for modulated signaling, essential for diverse behaviors. In vertebrates, synapses diversified further, with mammals developing intricate synaptic plasticity for advanced cognition. For instance, the human brain’s trillions of synapses support abstract thinking, far beyond the simpler setups in invertebrates.
Throughout evolution, synapses adapted to environmental pressures, like the giant synapses in squids for swift escapes. This progression underscores how synaptic complexity correlates with behavioral sophistication, bridging gaps from instinctual reactions to learned adaptations.
FAQ 9: What Are the Latest Advances in Synapse Research as of 2025?
Synapse research in 2025 has seen groundbreaking developments, from mapping entire brain connectomes to engineering artificial synapses for neuromorphic computing. These strides enhance our grasp of neural communication and open doors to novel therapies.
Notable progress includes:
- High-resolution imaging techniques that track synaptic changes in real-time, revealing how proteins like dystroglycan influence inhibitory synapse development.
- AI-driven models for synapse detection in large-scale brain data, improving accuracy in challenging regions and aiding connectome studies.
- Discoveries challenging old assumptions, such as distinct transmission sites for spontaneous versus evoked synaptic events, reshaping neuroscience fundamentals.
- Prototypes of energy-efficient artificial synapses that mimic human brain processing, potentially revolutionizing AI hardware.
- Methods to map brain-wide synaptic protein alterations, crucial for understanding disorders like Alzheimer’s.
These innovations promise targeted interventions for synaptic-related diseases and deeper insights into cognition.
FAQ 10: How Do Synapses Influence Everyday Functions in the Human Body?
Synapses play a starring role in routine bodily operations, coordinating everything from breathing to decision-making without us noticing. In sensory systems, they process inputs like light hitting the retina, where electrical synapses synchronize signals for clear vision. Motor functions rely on chemical synapses at neuromuscular junctions, where acetylcholine triggers muscle contractions for actions as simple as walking or smiling.
Internally, synapses regulate autonomic processes: norepinephrine synapses accelerate heart rate during excitement, while inhibitory ones calm it during rest. In digestion, they signal gut muscles to contract, aiding food movement. Even sleep involves synaptic shifts, with GABA promoting relaxation by dampening activity.
Cognitively, synapses enable quick thinking—integrating inputs to form responses, like dodging an obstacle. Over time, their plasticity refines habits, turning repeated actions into automatic routines. Overall, synapses ensure seamless harmony between mind and body, adapting to daily demands effortlessly.
FAQ 11: How Do Synapses Form and Develop in the Brain During Embryonic Stages?
Synapse formation is a remarkable process that begins early in embryonic development, laying the foundation for the intricate neural networks that enable thought, movement, and sensation. It starts around the third week of gestation when the neural tube forms, the precursor to the central nervous system. As neurons proliferate through neurogenesis, they migrate to their designated positions in the brain, guided by molecular signals. By the end of the embryonic period, around eight weeks, the basic structures of the brain are in place, and initial synaptic connections begin to emerge. These early synapses are often electrical at first, providing quick but simple communication, before chemical synapses dominate for more nuanced signaling.
As development progresses into the fetal stage, synaptogenesis ramps up dramatically. Millions of neurons start forming synapses, with peaks occurring between the second and third trimesters. The presynaptic neuron extends its axon toward the postsynaptic cell, where specialized proteins help establish contact across the synaptic cleft. Calcium signaling plays a pivotal role here, much like in mature synapses, triggering vesicle formation and neurotransmitter release. Environmental factors within the womb, such as maternal nutrition and stress levels, can influence this process, potentially affecting long-term brain health. By birth, a baby’s brain has about 100 billion neurons and trillions of synapses, though many are pruned later to refine circuits.
This overproduction and subsequent refinement ensure adaptability. For example, fetal movements like kicking stimulate synaptic growth, strengthening connections needed for motor skills. Genetic factors orchestrate much of this, but external cues fine-tune it. Disruptions, such as exposure to toxins, can lead to developmental disorders by impairing synapse maturation. Understanding these stages highlights why early prenatal care is crucial for optimal brain wiring, setting the stage for lifelong learning and function.
FAQ 12: What Is the Role of Glial Cells in Synaptic Signaling and Function?
Glial cells, often overlooked compared to neurons, are vital partners in synaptic activity, influencing everything from synapse formation to ongoing communication. The table below explores their diverse roles, drawing from recent insights into how they support and modulate neural networks.
| Glial Cell Type | Primary Role in Synapses | Key Mechanisms | Impact on Signaling | Examples in Health and Disease |
|---|---|---|---|---|
| Astrocytes | Regulate synapse formation and plasticity | Wrap around synapses, release gliotransmitters like ATP, control ion balance | Modulate neurotransmitter levels, enhance or inhibit transmission | Promote learning; dysfunction linked to Alzheimer’s synaptic loss |
| Microglia | Prune unnecessary synapses | Survey environment, engulf weak synapses via phagocytosis | Refine circuits, prevent overexcitation | Essential for development; overactivity in neuroinflammation |
| Oligodendrocytes | Myelinate axons for faster signaling | Form myelin sheaths, support synaptic stability | Speed up action potentials, influence timing | Myelination defects cause multiple sclerosis synaptic issues |
| Schwann Cells (peripheral) | Insulate peripheral synapses | Similar to oligodendrocytes but in PNS | Facilitate rapid peripheral transmission | Damage leads to neuropathy affecting sensory synapses |
| Radial Glia (developmental) | Guide neuron migration for synapse setup | Act as scaffolds during embryogenesis | Ensure proper positioning for connections | Mutations disrupt early synaptic architecture |
| Satellite Glia | Surround sensory neuron bodies | Modulate pain signaling at synapses | Regulate excitability in ganglia | Involved in chronic pain syndromes |
| Enteric Glia | Support gut-brain axis synapses | Regulate neurotransmitter release in ENS | Influence digestion-related signaling | Dysregulation in gastrointestinal disorders |
| Bergmann Glia (cerebellum) | Maintain Purkinje cell synapses | Clear excess glutamate, provide structural support | Prevent excitotoxicity, aid coordination | Impairments affect motor learning |
| Müller Glia (retina) | Sustain visual synapses | Recycle neurotransmitters, provide metabolic support | Enhance light signal processing | Degeneration in retinal diseases like glaucoma |
| Ependymal Cells | Line ventricles, influence CSF-synapse interactions | Cilia movement affects neurochemical flow | Indirectly modulate synaptic environment | Alterations in hydrocephalus impact signaling |
| Perisynaptic Schwann Cells | At neuromuscular junctions | Regulate acetylcholine release and uptake | Fine-tune muscle contraction signals | Key in regeneration after injury |
This comprehensive overview shows glial cells as active sculptors of synaptic environments, far beyond mere support, with implications for treating neurological conditions.
FAQ 13: How Does Aging Affect Synaptic Function and Signaling in the Brain?
Aging brings gradual changes to synaptic function, impacting how neurons communicate and process information. As we grow older, synaptic density often decreases, particularly in regions like the hippocampus, leading to slower signal transmission and reduced plasticity.
Key effects include:
- Diminished neurotransmitter release, where calcium signaling weakens, affecting action potential propagation and vesicle fusion.
- Increased oxidative stress, damaging synaptic proteins and membranes, which can impair receptor binding and ion channel function.
- Altered glial support, with astrocytes and microglia becoming less efficient at clearing debris, contributing to inflammation and synaptic loss.
- Reduced mitochondrial efficiency at synapses, lowering energy for signaling and heightening vulnerability to degeneration.
- Changes in synaptic plasticity mechanisms, like weakened long-term potentiation, making learning and memory formation harder.
These shifts can manifest as cognitive decline, but not inevitably—many older adults maintain robust synaptic health through active lifestyles.
FAQ 14: Can Lifestyle Factors Like Diet and Exercise Improve Synaptic Health?
Lifestyle choices profoundly influence synaptic health, offering accessible ways to bolster neural communication and resilience. Regular exercise, for instance, boosts blood flow to the brain, enhancing nutrient delivery and stimulating the release of growth factors like BDNF, which supports synapse formation and plasticity. Aerobic activities such as running or swimming have been shown to increase synaptic density in key areas like the hippocampus, aiding memory and mood regulation. Even moderate exercise can counteract age-related synaptic decline by reducing inflammation and promoting efficient signaling.
Diet plays an equally vital role, with nutrient-rich foods providing the building blocks for neurotransmitters and synaptic membranes. Omega-3 fatty acids from fish or nuts strengthen synaptic structures, while antioxidants in fruits and vegetables combat oxidative stress that damages synapses. A balanced diet low in processed sugars helps maintain stable energy for synaptic activity, preventing disruptions from blood sugar fluctuations. Combining diet with exercise amplifies benefits, as seen in studies where such interventions reversed some synaptic impairments in models of neurodegeneration.
Sleep and stress management round out these factors, as quality rest consolidates synaptic changes from daily experiences, and low stress preserves cortisol levels that otherwise erode synapses. Overall, adopting these habits early can foster lifelong synaptic vitality, potentially delaying or mitigating disorders linked to signaling breakdowns.
FAQ 15: What Are Neuromodulators and How Do They Differ from Neurotransmitters in Synaptic Function?
Neuromodulators and neurotransmitters both facilitate synaptic communication but operate differently, adding layers to neural signaling. The table below compares them, highlighting their roles, mechanisms, and impacts.
| Aspect | Neuromodulators | Neurotransmitters |
|---|---|---|
| Definition | Substances that alter synaptic efficacy without direct ion channel activation | Chemicals that directly transmit signals across synapses |
| Release Mechanism | Diffuse volume transmission, affecting broad areas | Targeted release into synaptic cleft |
| Duration of Effect | Longer-lasting, modulating over minutes to hours | Short-term, milliseconds to seconds |
| Primary Function | Enhance or suppress neurotransmitter effects, influence plasticity | Excite or inhibit postsynaptic cells via receptors |
| Examples | Dopamine, serotonin (in modulatory roles), neuropeptides like endorphins | Glutamate, GABA, acetylcholine |
| Synaptic Impact | Adjust sensitivity, promote learning by changing receptor density | Trigger immediate action potentials or inhibitions |
| Differences in Action | Act via second messengers, often G-protein coupled | Often ligand-gated ion channels for fast response |
| Role in Disorders | Imbalances in mood disorders, addiction | Deficits in epilepsy, Parkinson’s |
| Evolutionary Notes | Allow flexible network reconfiguration | Enable precise, rapid communication |
| Interaction Example | Serotonin modulates glutamate release for mood | Glutamate directly excites neurons for cognition |
This distinction underscores how neuromodulators provide fine-tuning, complementing the direct actions of neurotransmitters for complex behaviors.
FAQ 16: How Do Synapses Contribute to Sensory Perception in the Nervous System?
Synapses are central to transforming raw sensory inputs into meaningful perceptions, acting as gateways where information is filtered and integrated. In sensory pathways, like vision or touch, initial signals from receptors synapse with neurons in relay stations, such as the thalamus, before reaching the cortex for processing.
Critical contributions include:
- Signal amplification, where excitatory synapses boost weak inputs for detection.
- Integration of modalities, combining touch and sight via converging synapses for richer experiences.
- Adaptation through plasticity, adjusting synaptic strength based on repeated stimuli to prevent overload.
- Inhibition to sharpen focus, suppressing irrelevant signals for clarity.
- Ribbon synapses in senses like hearing, enabling continuous release for precise timing.
These processes ensure perceptions are accurate and adaptive, from detecting a whisper to appreciating a sunset.
FAQ 17: What Is Synaptic Pruning and Why Is It Important for Brain Development?
Synaptic pruning is the brain’s natural process of eliminating excess synapses, refining neural circuits for efficiency. It begins in infancy, peaking between ages 2 and 10, where up to half of early synapses are removed based on usage. This “use it or lose it” mechanism strengthens frequently activated pathways while discarding underused ones, optimizing energy and space.
The importance lies in sculpting a mature brain capable of focused thinking and quick responses. Without pruning, circuits could become noisy and inefficient, hindering learning. Microglia drive this by engulfing weak synapses, guided by activity levels. In adolescence, pruning continues in prefrontal areas, aiding executive functions like decision-making.
Abnormal pruning links to conditions like autism (insufficient) or schizophrenia (excessive), emphasizing its role in mental health. Overall, it transforms the overconnected infant brain into a streamlined adult one, enabling advanced cognition.
FAQ 18: What Laboratory Techniques Are Used to Study Synapses in 2025?
Advancements in 2025 have expanded tools for synapse research, from imaging to molecular analysis. The table details key techniques, their applications, and recent innovations.
| Technique | Description | Primary Use | Advantages | Recent Developments (2025) |
|---|---|---|---|---|
| Electron Microscopy | High-resolution imaging of synaptic structures | Visualize cleft, vesicles | Ultra-detailed views | Enhanced with AI for 3D reconstructions |
| Optogenetics | Light-activated control of neurons | Manipulate synaptic activity | Precise temporal control | Combined with CRISPR for targeted edits |
| Patch-Clamp Electrophysiology | Record electrical activity at synapses | Measure currents, potentials | Real-time functional data | Miniaturized for in vivo studies |
| Synaptic Protein Mapping (e.g., DELTA) | Track protein turnover brain-wide | Study plasticity changes | Single-synapse resolution | Uses fluorescent dyes for dynamic imaging |
| Multipatch Recordings | Simultaneous patching of multiple neurons | Analyze connectivity | Catalogs thousands of synapses | Applied to human tissue samples |
| CRISPR-Based Tools | Edit genes affecting synapses | Model disorders | Specific interventions | New variants target synaptic repair |
| Two-Photon Microscopy | Live imaging of deep brain synapses | Observe in behaving animals | Non-invasive depth penetration | Improved resolution for memory studies |
| SynPull | Isolate synaptic proteins | Characterize aggregates | High-detail proteomics | Validated for neurodegeneration research |
| Artificial Synapses | Lab-grown models | Test computations | Mimic brain efficiency | Used in neuromorphic computing |
| Connectomics | Map entire synaptic networks | Understand wiring | Comprehensive overviews | Faster with machine learning |
These methods drive deeper insights into synaptic mechanics and therapies.
FAQ 19: What Emerging Therapies Target Synaptic Disorders as of 2025?
Emerging therapies in 2025 focus on restoring synaptic function, offering hope for conditions like Alzheimer’s and ALS. Innovations include drugs enhancing synaptic resilience and gene edits repairing connections.
Promising approaches encompass:
- Synaptic regenerative pills, like SPG302, that promote synapse regrowth in neurodegenerative diseases.
- CRISPR technologies delivering RNA to damaged neurons, stimulating regeneration in ALS models.
- Protein-targeting drugs, such as those binding new sites on synaptic proteins to control seizures.
- BDNF-boosting agents, like ketamine derivatives, restoring plasticity in depression.
- Precision neurotherapeutics from startups, focusing on synapse repair for CNS disorders.
These therapies aim to halt progression by directly addressing synaptic loss.
FAQ 20: How Do Environmental Toxins Affect Synaptic Signaling in the Nervous System?
Environmental toxins pose significant threats to synaptic signaling, disrupting the delicate balance of neural communication. Pesticides, for example, can inhibit enzymes like acetylcholinesterase, leading to neurotransmitter buildup and overexcitation, potentially causing seizures or developmental delays. Heavy metals such as lead interfere with calcium signaling, impairing vesicle release and synaptic plasticity, which is particularly harmful during brain development.
Other toxins, like industrial chemicals, induce oxidative stress, damaging synaptic membranes and receptors, reducing signal efficiency. Microplastics and air pollutants trigger neuroinflammation, where activated microglia prune healthy synapses excessively. Chronic exposure can mimic aging effects, accelerating cognitive decline by weakening synaptic integration.
Mitigation involves reducing exposure through regulations and personal choices, as early interventions can sometimes reverse mild damage, underscoring the need for environmental awareness to protect synaptic health.
Acknowledgement
The Examsmeta.com website expresses its sincere gratitude to the wealth of scientific knowledge provided by several reputable sources that greatly enriched the content of the article “Understanding Synaptic Signaling: The Vital Communication Network of Neurons.” The detailed insights and cutting-edge research shared by these platforms were instrumental in crafting a comprehensive and accessible exploration of synaptic signaling.
Specifically, acknowledges the contributions of National Institute of Neurological Disorders and Stroke (NINDS) for its authoritative resources on neurological mechanisms, Nature (Nature) for its peer-reviewed studies on synaptic plasticity and neuroscience advancements, ScienceDirect (ScienceDirect) for its extensive database of synaptic research articles, and PubMed (PubMed) for providing access to critical primary research papers.
These sources ensured the article’s accuracy and depth, making complex concepts approachable for all readers.
Key contributions from these sources include:
- NINDS: Offered foundational explanations of synaptic function and disorders, grounding the article in reliable public health information.
- Nature: Provided recent findings on synaptic plasticity and neuromodulation, enhancing the discussion on learning and memory.
- ScienceDirect: Supplied detailed studies on glial roles and synaptic development, adding depth to technical sections.
- PubMed: Enabled access to primary research on emerging synaptic therapies and environmental impacts, ensuring up-to-date content.

