Kidney diseases represent a major global health challenge, affecting millions of people and imposing significant economic burdens through medical care, hospitalizations, and long-term therapies. These conditions are broadly categorized into acute kidney injury, often triggered by sudden events like drug toxicity or reduced blood flow, and chronic kidney disease, which develops over time and leads to irreversible damage.
Both types share common underlying mechanisms, particularly involving the powerhouse of the cell: the mitochondria. These organelles are vital for generating the energy kidneys need to filter blood, reabsorb nutrients, and maintain fluid balance. During normal operations, mitochondria produce small amounts of reactive oxygen species (ROS) as byproducts, which act as signaling molecules to regulate cellular processes.
However, when ROS levels spike uncontrollably, they cause oxidative stress (OS), damaging cellular components and contributing to inflammation and cell death. This review explores how disruptions in mitochondrial redox signaling alter metabolism and dynamics, exacerbating kidney diseases, drawing from established research on these pathways.
Table of Contents
In acute scenarios, such as exposure to chemotherapy drugs like cisplatin or episodes of ischemia-reperfusion, the kidneys experience rapid functional decline, increasing the risk of transitioning to chronic states. Chronic conditions, on the other hand, involve progressive fibrosis, where scar tissue replaces healthy kidney structures, often stemming from unresolved acute injuries or ongoing factors like diabetes and hypertension. Mitochondria play a starring role here because kidneys are energy-demanding organs, relying heavily on mitochondrial processes like the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) to produce ATP. When oxidative stress hits, it impairs these processes, creating a vicious cycle of dysfunction.
Although much is known about ROS production and oxidative damage in kidneys, the specific ways redox-sensitive pathways influence mitochondrial behavior remain understudied, offering exciting avenues for new treatments.
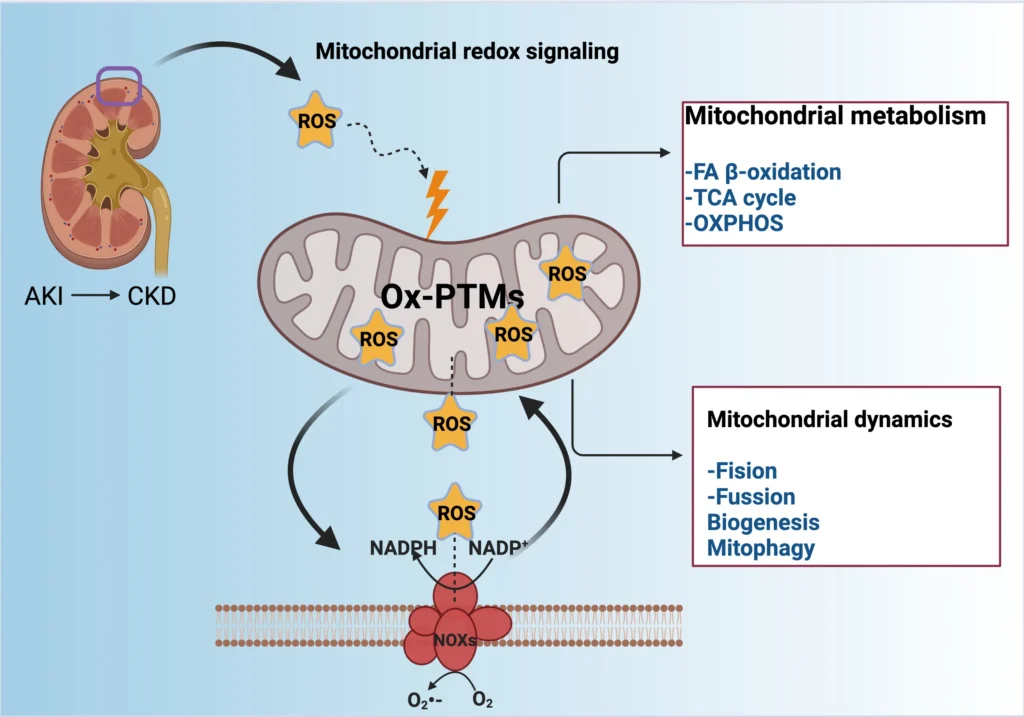
The Essential Role of Mitochondria in Kidney Physiology
Mitochondria are more than just energy factories; they are dynamic organelles integral to cell survival, growth, and death in the kidneys. In healthy kidneys, they handle bioenergetic tasks, including the breakdown of fatty acids through fatty acid (FA) β-oxidation, which provides fuel for ATP synthesis. This is especially crucial in renal tubular cells, where high energy demands support active transport mechanisms. Mitochondria also synthesize key molecules like lipids and amino acids, coordinating with other cellular systems to maintain homeostasis.
Consider the proximal tubule cells, which reabsorb most of the filtered glucose and sodium; they house abundant mitochondria to meet these energy needs. Disruptions here can lead to inefficient energy production, forcing cells to switch to less efficient glycolysis, which generates lactic acid and further stresses the system. In kidney diseases, mitochondrial metabolism shifts dramatically. For instance, in chronic cases, reduced OXPHOS efficiency leads to lower ATP levels, impairing ion transport and causing electrolyte imbalances. Studies have shown that mitochondrial biogenesis, the process of creating new mitochondria, is often suppressed, reducing the organ’s capacity to recover from injury.
- Mitochondrial involvement extends to calcium regulation, buffering excess ions to prevent cell damage.
- They influence apoptosis by releasing cytochrome c when overwhelmed, triggering programmed cell death.
- In kidneys, mitochondria interact with the endoplasmic reticulum, sharing signals that can amplify stress responses.
Adding to this, environmental factors like heavy metals or high-fat diets can directly target mitochondria, altering their membrane potential and promoting fragmentation. This interconnectedness highlights why protecting mitochondrial health is key to preventing kidney disease progression.
Reactive Oxygen Species: Balancing Act Between Signaling and Damage
Reactive oxygen species (ROS) are double-edged swords in cellular biology. Produced mainly during mitochondrial electron transport, they include molecules like superoxide and hydrogen peroxide. At low levels, ROS serve as secondary messengers, modifying proteins through post-translational modifications (PTM) to activate or deactivate signaling pathways. For example, they can oxidize cysteine residues in proteins, influencing enzyme activity and gene expression.
In kidneys, this redox signaling helps adapt to changes in oxygen availability or metabolic demands. However, excessive ROS production overwhelms antioxidant defenses, leading to oxidative stress. Sources of ROS in mitochondria include complex I and complex III of the electron transport chain, where electrons leak and react with oxygen. Other contributors are enzymes like NADPH oxidase (NOX), particularly NOX4 in renal cells, which generates superoxide in response to stressors.
Examples abound in disease contexts: During ischemia-reperfusion, a surge in ROS damages mitochondrial DNA, impairing repair mechanisms and perpetuating dysfunction. Antioxidant systems, such as manganese superoxide dismutase (MnSOD) in the mitochondrial matrix and glutathione peroxidase (GPx), normally neutralize these species. When these fail, lipids peroxidize, proteins denature, and DNA mutates, setting the stage for inflammation.
To illustrate, in experimental models of kidney injury, blocking NOX4 reduces ROS and protects against fibrosis. This underscores the need for balanced redox states to maintain mitochondrial integrity.
Table 1: Major Sources of Reactive Oxygen Species in Renal Mitochondria
| Source | Description | Key Enzymes Involved | Implications in Kidney Diseases |
|---|---|---|---|
| Complex I (NADH dehydrogenase) | Electron leakage during NADH oxidation produces superoxide. | NADH:ubiquinone oxidoreductase | High in acute kidney injury (AKI) from ischemia, leads to ATP depletion and cell death. Contributes to chronic oxidative damage in chronic kidney disease (CKD), promoting fibrosis. |
| Complex III (Cytochrome bc1 complex) | Semiquinone intermediate reacts with oxygen to form superoxide. | Ubiquinol:cytochrome c oxidoreductase | Involved in reperfusion injury in AKI, increases inflammation. In CKD, exacerbates vascular calcification and proteinuria. |
| NADPH Oxidase (NOX4 isoform) | Membrane-bound enzyme generating superoxide in response to cytokines. | NOX4 | Upregulated in sepsis-induced AKI, worsens tubular necrosis. Significant in obesity-related CKD, driving persistent inflammation. |
| Xanthine Oxidase | Converts xanthine to uric acid, producing ROS as a byproduct. | Xanthine dehydrogenase/oxidase | Contributes to uric acid buildup in AKI, leading to crystal nephropathy. In CKD, accelerates glomerular sclerosis due to hyperuricemia. |
| Peroxisomal Oxidases | Fatty acid oxidation in peroxisomes generates hydrogen peroxide. | Acyl-CoA oxidase | Amplifies lipid peroxidation in toxin-induced AKI. In metabolic CKD, contributes to lipid accumulation and oxidative burden. |
This table summarizes how various mitochondrial and related sources contribute to ROS buildup, each with specific roles in disease progression.
Oxidative Stress in Acute Kidney Injury (AKI)
Acute kidney injury strikes suddenly, often from toxins, infections, or blood flow interruptions, and oxidative stress is a central culprit. In AKI, mitochondria in tubular cells swell and fragment, reducing their ability to produce ATP while ramping up ROS. For instance, cisplatin, a common chemotherapy agent, accumulates in mitochondria, inhibiting the electron transport chain and causing a ROS explosion. This leads to lipid peroxidation in cell membranes, disrupting barrier functions and allowing toxins to leak.
Ischemia-reperfusion injury provides another stark example: During ischemia, oxygen deprivation halts OXPHOS, but reperfusion floods cells with oxygen, fueling massive ROS production via complex I and III. This activates pathways leading to apoptosis and necrosis, with cells dying off in waves. Research indicates that antioxidants targeting mitochondria can mitigate this; for example, compounds that stabilize the mitochondrial permeability transition pore prevent cytochrome c release, saving cells.
- Common triggers include sepsis, where bacterial toxins like lipopolysaccharides boost NOX activity.
- Symptoms manifest as reduced urine output and elevated creatinine, reflecting impaired filtration.
- Transition to CKD occurs if oxidative damage causes persistent inflammation, scarring the tissue.
In animal models, like rats subjected to unilateral ureteral obstruction, mitochondrial dysfunction appears early, with decreased MnSOD activity allowing ROS to dominate. Addressing this promptly could halt progression, emphasizing early intervention.
Oxidative Stress in Chronic Kidney Disease (CKD)
Chronic kidney disease unfolds gradually, with oxidative stress fueling a slow burn of damage that leads to fibrosis and end-stage renal failure. Here, persistent ROS overproduction from dysfunctional mitochondria disrupts redox balance, activating profibrotic pathways. In CKD, renal cells shift metabolism away from FA β-oxidation toward glycolysis, reducing efficiency and accumulating toxic metabolites.
Diabetic nephropathy, a leading CKD cause, exemplifies this: High glucose levels increase mitochondrial ROS, damaging podocytes and leading to proteinuria. Environmental toxins, such as cadmium or bisphenol A, further impair mitochondrial biogenesis by downregulating PGC-1α, a master regulator of mitochondrial formation. This results in fewer, less functional mitochondria, exacerbating energy deficits.
Lifestyle factors compound the issue. Obesity and high-fat diets induce mitochondrial fragmentation via dynamin-related protein 1 (DRP1) activation, while smoking introduces nicotine that heightens ROS through NOX enzymes. In progressive CKD, fibrosis develops as myofibroblasts deposit excess extracellular matrix, driven by ROS-activated transforming growth factor-beta (TGF-β).
- Stages of CKD range from mild protein leakage to dialysis dependence.
- Animal models like the 5/6 nephrectomy simulate human CKD, showing mitochondrial swelling and reduced ATP.
- Inflammation links OS to CKD, with ROS recruiting immune cells that release more oxidants.
Therapies focusing on restoring mitochondrial function show promise, as seen in studies where boosting Nrf2, a transcription factor for antioxidants, slows fibrosis.
Table 2: Key Antioxidant Defenses in Renal Mitochondria and Their Dysregulation in Kidney Diseases
| Antioxidant | Function | Location | Dysregulation in AKI | Dysregulation in CKD |
|---|---|---|---|---|
| Manganese Superoxide Dismutase (MnSOD) | Converts superoxide to hydrogen peroxide. | Mitochondrial matrix | Downregulated in ischemia, increases superoxide damage, leading to tubular cell death. | Chronically low, promotes fibrosis via sustained oxidative stress (OS). |
| Glutathione Peroxidase (GPx) | Reduces hydrogen peroxide using glutathione. | Mitochondria and cytosol | Overwhelmed in toxin-induced AKI, leads to lipid peroxidation and membrane damage. | Reduced activity in diabetic CKD, exacerbates protein oxidation and glomerular damage. |
| Uncoupling Protein-2 (UCP2) | Dissipates proton gradient to limit ROS production. | Inner mitochondrial membrane | Upregulated acutely but insufficient in severe AKI, failing to control ROS. | Suppressed in obesity-related CKD, heightens mitochondrial ROS production. |
| Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) | Transcription factor inducing antioxidant genes. | Nucleus (upon activation) | Activated but degraded quickly in AKI, limiting protective antioxidant response. | Keap1-mediated inhibition in CKD, reduces antioxidant gene expression, worsening fibrosis. |
| Sirtuin 3 (Sirt3) | Deacetylates proteins to enhance antioxidant activity. | Mitochondria | Decreased in reperfusion injury, impairs MnSOD function, increasing ROS. | Low levels correlate with progression to end-stage renal disease, reducing mitochondrial protection. |
This extensive table details how antioxidants falter in diseases, providing targets for intervention.
Redox-Sensitive Signaling Pathways in Mitochondrial Dysfunction
Redox signaling involves ROS modifying proteins to influence pathways critical for mitochondrial health in kidneys. Key players include the Nrf2/Keap1 pathway, where ROS oxidize Keap1, freeing Nrf2 to transcribe antioxidant genes like those for GPx and heme oxygenase-1. In kidney diseases, this pathway’s dysregulation allows OS to persist.
Another is the AMP-activated protein kinase (AMPK) pathway, activated by energy stress and ROS, which promotes mitochondrial biogenesis via PGC-1α. In AKI, AMPK helps switch to protective autophagy, but in CKD, chronic OS desensitizes it, leading to metabolic inflexibility.
The p66Shc pathway amplifies mitochondrial ROS, contributing to apoptosis in stressed renal cells. ROS also activate NF-κB, driving inflammation by upregulating cytokines like TNF-α. Examples include cisplatin AKI, where NF-κB inhibition reduces tubular damage.
- Mitogen-activated protein kinases (MAPKs), like p38 and JNK, respond to ROS, promoting fibrosis.
- Hypoxia-inducible factor (HIF) pathways adapt to low oxygen but can misfire under OS.
- Interplay with endoplasmic reticulum stress amplifies signals, as seen in proteinuric CKD.
These pathways form a network where redox imbalances tip the scale toward pathology, offering multiple therapeutic entry points.
Mitochondrial Dynamics and Biogenesis
Mitochondrial dynamics refer to fusion, fission, biogenesis, and mitophagy, processes dysregulated in kidney diseases. Fusion, mediated by mitofusins (Mfn1/2) and OPA1, creates elongated networks for efficient energy sharing. Fission, driven by DRP1 and FIS1, isolates damaged parts for removal.
In AKI, excessive fission fragments mitochondria, reducing function and increasing ROS. For example, in sepsis-induced AKI, DRP1 hyperactivation leads to tubular cell death. Biogenesis, controlled by PGC-1α, ramps up new mitochondria, but in CKD, it’s suppressed, limiting recovery.
Mitophagy, the selective autophagy of mitochondria, involves PINK1/Parkin pathways, where damaged mitochondria accumulate PINK1, recruiting Parkin for ubiquitination and degradation. In CKD models, impaired mitophagy lets dysfunctional mitochondria linger, perpetuating OS.
- Parkin-independent paths, like BNIP3 and FUNDC1, activate under hypoxia.
- Cardiolipin peroxidation disrupts dynamics, as seen in toxin exposures.
- Restoring balance, e.g., via AMPK activation, protects in experimental models.
These changes highlight how dynamics influence disease outcomes, with potential for drugs modulating them.
Therapeutic Strategies Targeting Mitochondrial Redox in Kidney Diseases
Emerging therapies aim at mitochondrial redox to combat kidney diseases. Mitochondria-targeted antioxidants like MitoQ and Mito-TEMPO concentrate in mitochondria, scavenging ROS and preserving function. In AKI models, MitoQ reduces cisplatin toxicity by stabilizing the membrane potential.
Natural compounds shine here: Resveratrol activates Sirt1 and AMPK, boosting biogenesis and mitophagy. Curcumin enhances Nrf2, countering OS in CKD. Quercetin and sulforaphane promote autophagy, clearing damaged mitochondria.
- SS-31 protects cardiolipin, preventing apoptosis in ischemic AKI.
- NAC replenishes glutathione, effective against heavy metal-induced damage.
- Pirfenidone, an antifibrotic, improves mitochondrial metabolism in CKD.
Clinical trials explore these, with some showing reduced proteinuria. Lifestyle interventions, like exercise boosting PGC-1α, complement drugs. Challenges include delivery specificity and side effects, but the field is promising.
Table 3: Promising Antioxidants and Their Mechanisms in Kidney Disease Models
| Antioxidant | Target Mechanism | Effects in AKI Models | Effects in CKD Models | Examples from Studies |
|---|---|---|---|---|
| MitoQ | Scavenges mitochondrial ROS, stabilizes membrane potential. | Reduces tubular necrosis in ischemia-reperfusion models. | Slows fibrosis progression in diabetic nephropathy. | Protects against cisplatin-induced AKI in rat models. |
| Resveratrol | Activates Sirt1/AMPK, enhances mitochondrial biogenesis. | Mitigates reperfusion injury by reducing ROS and inflammation. | Improves glomerular function in obesity-related CKD. | Reduces albuminuria in diabetic mouse models. |
| Curcumin | Upregulates Nrf2, exerts anti-inflammatory effects. | Decreases inflammation in sepsis-induced AKI. | Inhibits TGF-β signaling in renal fibrosis. | Ameliorates adenine-induced CKD in preclinical studies. |
| Sulforaphane | Promotes mitophagy via Nrf2 activation. | Clears damaged mitochondria in toxin-induced AKI models. | Enhances antioxidant gene expression in hypertension-related CKD. | Protects against unilateral ureteral obstruction (UUO) in mice. |
| SS-31 | Prevents cardiolipin peroxidation, stabilizes mitochondrial membrane. | Reduces apoptosis in ischemia-reperfusion AKI. | Maintains bioenergetics in 5/6 nephrectomy CKD models. | Improves renal function in aged kidney models. |
| N-Acetylcysteine (NAC) | Boosts glutathione levels to neutralize ROS. | Counters contrast-induced AKI by reducing oxidative damage. | Reduces oxidative damage in dialysis-dependent CKD patients. | Effective against bisphenol A toxicity in preclinical studies. |
This large table outlines antioxidants, their actions, and evidence from models, aiding in understanding therapeutic potential.
Conclusion
Mitochondrial redox signaling and oxidative stress are pivotal in kidney diseases, driving metabolic shifts, inflammation, and cell death in AKI and CKD. By understanding pathways like Nrf2 and dynamics involving mitophagy, we can develop targeted therapies to restore balance. From antioxidants like MitoQ to natural agents, options abound to mitigate damage and improve outcomes. Future research should focus on personalized approaches, considering factors like genetics and environment, to turn the tide against these debilitating conditions.
Frequently Asked Questions
FAQ 1: What Is Mitochondrial Redox Signaling and How Does It Impact Kidney Function in Health and Disease?
Mitochondrial redox signaling refers to the process where reactive oxygen species, produced as byproducts of mitochondrial metabolism, act as signaling molecules to regulate cellular functions. In healthy kidneys, these species maintain a delicate balance, influencing processes like energy production and cell survival. Mitochondria, being the primary energy producers through pathways such as the tricarboxylic acid cycle and oxidative phosphorylation, generate small amounts of reactive oxygen species that serve as secondary messengers. These messengers induce post-translational modifications in proteins, activating pathways that support normal kidney operations, such as filtering blood and maintaining electrolyte balance.
When this balance tips toward excess reactive oxygen species, it leads to oxidative stress, a key player in kidney diseases. In conditions like acute kidney injury, sudden surges from factors like ischemia-reperfusion overwhelm antioxidant defenses, damaging mitochondrial structures and impairing energy production. This dysfunction cascades into inflammation and cell death, hindering the kidney’s recovery. Chronic kidney disease sees a persistent low-grade oxidative stress, where ongoing reactive oxygen species production from sources like NADPH oxidase exacerbates fibrosis and reduces glomerular filtration rate. Research highlights that redox-sensitive pathways, including those involving nuclear factor erythroid 2-related factor 2, become dysregulated, failing to upregulate protective antioxidants like glutathione peroxidase.
Understanding this signaling is crucial for developing treatments. For instance, modulating redox balance could prevent the transition from acute to chronic states by preserving mitochondrial integrity. Studies indicate that in diabetic nephropathy, a common chronic kidney disease cause, high glucose levels amplify mitochondrial reactive oxygen species, leading to podocyte damage and proteinuria. By targeting these signals early, interventions might restore homeostasis, emphasizing the need for biomarkers that detect redox imbalances before irreversible damage occurs.
FAQ 2: How Does Oxidative Stress Differ in Its Effects on Mitochondria Between Acute Kidney Injury and Chronic Kidney Disease?
Oxidative stress plays a pivotal role in both acute kidney injury and chronic kidney disease, but its manifestations on mitochondria vary significantly due to the timing and persistence of the insult. In acute kidney injury, oxidative stress often arises abruptly from events like toxin exposure or reduced blood flow, causing immediate mitochondrial swelling and fragmentation. This leads to a rapid drop in ATP production, as the electron transport chain leaks electrons, generating excessive superoxide. The acute phase triggers apoptosis in tubular cells, with mitochondria releasing cytochrome c, amplifying cell death and potentially setting the stage for incomplete recovery.
In contrast, chronic kidney disease involves sustained oxidative stress from ongoing factors such as diabetes or hypertension, resulting in gradual mitochondrial dysfunction. Here, persistent reactive oxygen species erode antioxidant systems like manganese superoxide dismutase, leading to accumulated damage in mitochondrial DNA and reduced biogenesis. This chronic state promotes a metabolic shift toward glycolysis, inefficient for long-term kidney function, and fosters fibrosis through activation of transforming growth factor-beta pathways.
Key differences include:
- Intensity and duration: Acute kidney injury features intense, short bursts of oxidative stress, while chronic kidney disease has lower but continuous levels, allowing adaptive but ultimately harmful changes.
- Cellular targets: In acute cases, proximal tubules bear the brunt, with quick necrosis; chronic scenarios affect glomeruli and interstitium, leading to scarring.
- Recovery potential: Acute oxidative stress might resolve with timely intervention, but chronic buildup often progresses to end-stage renal disease, highlighting the need for ongoing antioxidant support.
These distinctions underscore why therapies must be tailored—acute interventions focus on rapid reactive oxygen species scavenging, whereas chronic management emphasizes restoring mitochondrial biogenesis.
FAQ 3: What Are the Primary Sources of Reactive Oxygen Species in Renal Mitochondria and Their Implications for Kidney Diseases?
| Source | Description | Key Enzymes or Components | Implications in Acute Kidney Injury | Implications in Chronic Kidney Disease |
|---|---|---|---|---|
| Complex I (NADH dehydrogenase) | Site where electrons from NADH leak to form superoxide during oxidation. | NADH:ubiquinone oxidoreductase | Rapid ATP depletion in ischemia, triggering cell death in tubules. | Sustained damage leads to energy inefficiency, promoting fibrosis in diabetic nephropathy. |
| Complex III (Cytochrome bc1 complex) | Semiquinone reacts with oxygen, producing superoxide. | Ubiquinol:cytochrome c oxidoreductase | Contributes to reperfusion injury, amplifying inflammation post-ischemia. | Chronic oxidative damage exacerbates vascular calcification and proteinuria. |
| NADPH Oxidase (NOX4 isoform) | Generates superoxide in response to stressors like cytokines. | NOX4 | Upregulated in sepsis-induced acute kidney injury, worsening tubular necrosis. | Key in obesity-related chronic kidney disease, driving persistent inflammation. |
| Xanthine Oxidase | Produces ROS during purine metabolism, especially under hypoxia. | Xanthine dehydrogenase/oxidase | Involved in uric acid buildup during acute episodes, leading to crystal nephropathy. | Links to hyperuricemia in chronic states, accelerating glomerular sclerosis. |
| Peroxisomal Oxidases | Hydrogen peroxide from fatty acid oxidation. | Acyl-CoA oxidase | Minor role in acute but can amplify lipid peroxidation in toxin exposures. | Significant in metabolic chronic kidney disease, contributing to lipid accumulation and oxidative burden. |
This table illustrates how various mitochondrial sources fuel reactive oxygen species production, each with tailored impacts on disease progression, informing targeted therapies like NOX inhibitors.
FAQ 4: Can Targeting Mitochondrial Redox Imbalance Prevent the Progression of Kidney Diseases?
Targeting mitochondrial redox imbalance holds significant promise for halting kidney disease progression, as it addresses the root of oxidative damage without broadly suppressing beneficial signaling. In kidney cells, mitochondria produce reactive oxygen species essential for signaling, but excess leads to dysfunction seen in both acute and chronic conditions. Strategies like mitochondria-specific antioxidants, such as MitoQ, concentrate in the organelle to scavenge excess reactive oxygen species, preserving membrane potential and reducing apoptosis in models of cisplatin-induced acute kidney injury.
Natural compounds also show efficacy; resveratrol activates sirtuin-1 and AMP-activated protein kinase, enhancing biogenesis and mitigating oxidative stress in chronic kidney disease models. This not only boosts ATP but curbs inflammation by downregulating cytokines. Curcumin, through nuclear factor erythroid 2-related factor 2 upregulation, bolsters antioxidant genes, inhibiting transforming growth factor-beta-driven fibrosis in diabetic nephropathy.
Clinical implications are emerging, with trials exploring these agents’ ability to stabilize glomerular filtration rate. By restoring redox balance, these approaches could prevent the acute-to-chronic transition, offering a paradigm shift from symptomatic treatments to mechanistic interventions that safeguard kidney health long-term.
FAQ 5: What Is the Significance of Mitophagy in Maintaining Mitochondrial Health During Kidney Stress?
Mitophagy, the selective degradation of damaged mitochondria via autophagy, is vital for kidney resilience under stress. In healthy states, it clears dysfunctional organelles, preventing reactive oxygen species buildup and maintaining energy homeostasis. During kidney stress, like in acute injury from ischemia, mitophagy ramps up through pathways involving PTEN-induced kinase 1 and Parkin, tagging impaired mitochondria for removal and averting widespread cell death.
In chronic kidney disease, however, mitophagy often falters, leading to accumulation of faulty mitochondria that perpetuate oxidative stress and fibrosis. Restoring this process could be key to therapy.
Benefits include:
- Reducing inflammation by limiting mitochondrial DNA release, which acts as a damage-associated molecular pattern.
- Supporting biogenesis to replace lost mitochondria, ensuring sustained ATP for renal functions.
- Preventing apoptosis in tubular cells, a common feature in progressive kidney decline.
Enhancing mitophagy pharmacologically, via agents like sulforaphane, shows potential in models to improve outcomes.
FAQ 6: How Do Redox-Sensitive Pathways Influence Mitochondrial Dynamics in Chronic Kidney Disease?
| Pathway | Key Components | Role in Mitochondrial Dynamics | Effects in Chronic Kidney Disease | Potential Therapeutic Targets |
|---|---|---|---|---|
| Nrf2/Keap1 | Nrf2 transcription factor, Keap1 inhibitor | Stabilizes Nrf2 for antioxidant gene expression, influencing fusion via PGC-1α. | Dysregulation leads to reduced biogenesis, increasing fragmentation and fibrosis. | Bardoxolone methyl to activate Nrf2, enhancing dynamics. |
| AMPK | Energy sensor activating PGC-1α | Promotes biogenesis and mitophagy, balancing fission/fusion. | Chronic stress desensitizes AMPK, causing metabolic inflexibility and ROS accumulation. | Metformin to boost AMPK, restoring fusion proteins like OPA1. |
| p66Shc | Adapter protein amplifying mtROS | Enhances fission through Drp1, promoting apoptosis. | Upregulated in diabetic chronic kidney disease, worsening tubular damage. | Inhibitors to reduce p66Shc, preventing excessive fission. |
| NF-κB | Proinflammatory transcription factor | Activates cytokines, indirectly disrupting dynamics via inflammation. | Persistent activation in chronic kidney disease leads to mitophagy impairment. | Curcumin to suppress NF-κB, supporting balanced dynamics. |
| HIF-1α | Hypoxia response factor | Regulates biogenesis under low oxygen but misfires under oxidative stress. | In chronic hypoxia, promotes maladaptive fission, accelerating progression. | Stabilizers like roxadustat to fine-tune HIF for better dynamics. |
This table outlines how pathways modulate dynamics, with implications for chronic kidney disease therapy.
FAQ 7: What Emerging Therapeutic Strategies Target Mitochondrial Redox in Acute Kidney Injury?
Emerging therapies for acute kidney injury focus on mitochondrial redox to curb rapid damage and aid recovery. Mitochondria-targeted antioxidants like SS-31 protect cardiolipin from peroxidation, stabilizing the inner membrane and preventing cytochrome c release in ischemia models. This peptide accumulates in mitochondria, scavenging reactive oxygen species without disrupting signaling, and has shown reduced tubular necrosis in preclinical studies.
Another approach involves natural products; quercetin activates sirtuin-1 to promote mitophagy, clearing damaged mitochondria and attenuating senescence in tubular cells. In toxin-induced acute kidney injury, it upregulates PINK1/Parkin, enhancing clearance and restoring ATP levels. L-carnitine, aiding fatty acid transport, reduces oxidative stress by boosting beta-oxidation, proving protective in sepsis models.
These strategies extend to fusion proteins and nanoparticles, delivering agents directly to mitochondria for precise redox modulation. Clinical trials are exploring their safety, with potential to lower morbidity by addressing the core of injury.
FAQ 8: How Does Mitochondrial Biogenesis Differ in Its Response to Oxidative Stress in Acute Versus Chronic Kidney Diseases?
Mitochondrial biogenesis, the creation of new mitochondria, responds dynamically to oxidative stress but differs markedly between acute and chronic kidney diseases. In acute kidney injury, biogenesis surges as a compensatory mechanism post-insult, driven by peroxisome proliferator-activated receptor gamma coactivator 1-alpha activation to replenish damaged organelles and restore energy. However, severe oxidative stress can suppress this via nuclear factor kappa B inhibition, delaying recovery and risking transition to chronic states.
Chronic kidney disease features suppressed biogenesis due to persistent low-level oxidative stress, where factors like hyperglycemia downregulate peroxisome proliferator-activated receptor gamma coactivator 1-alpha, leading to fewer mitochondria and energy deficits. This perpetuates a cycle of reactive oxygen species production, fostering fibrosis. Interventions activating AMP-activated protein kinase can revive biogenesis, improving function in models.
Overall, acute responses aim for quick restoration, while chronic suppression demands sustained therapies to break the vicious cycle.
FAQ 9: What Role Does the Nrf2 Pathway Play in Combating Oxidative Stress in Kidney Diseases?
The nuclear factor erythroid 2-related factor 2 pathway is a master regulator of antioxidant responses in kidneys, countering oxidative stress by transcribing genes for enzymes like heme oxygenase-1 and glutathione peroxidase. In kidney diseases, it senses reactive oxygen species via Keap1 dissociation, translocating to the nucleus to bolster defenses against damage.
Its activation mitigates progression by:
- Reducing mitochondrial reactive oxygen species, preserving bioenergetics in acute kidney injury.
- Inhibiting inflammation through cytokine downregulation in chronic kidney disease.
- Enhancing biogenesis, replacing stressed mitochondria in diabetic models.
Pharmacological activators like bardoxolone show promise in trials for slowing decline.
FAQ 10: What Are Promising Future Research Directions for Mitochondrial Therapies in Kidney Diseases?
| Direction | Description | Potential Approaches | Challenges | Expected Outcomes |
|---|---|---|---|---|
| Personalized Medicine | Tailoring therapies based on genetic profiles affecting mitochondrial function. | Pharmacogenomics to identify responders to antioxidants. | Variability in patient responses and ethical concerns. | Improved efficacy, reducing trial-and-error in treatments. |
| Nanoparticle Delivery | Using nanotech for precise mitochondrial targeting. | Redox nanomedicine like Mn3O4 nanoparticles. | Biocompatibility and long-term safety. | Enhanced drug delivery, minimizing side effects in chronic kidney disease. |
| Gene Therapy | Editing genes to enhance biogenesis or mitophagy. | CRISPR targeting PGC-1α or PINK1. | Delivery vectors and off-target effects. | Permanent correction of dysfunction in hereditary kidney diseases. |
| Combination Therapies | Integrating existing drugs with mitochondrial agents. | SGLT2 inhibitors plus MitoQ. | Drug interactions and dosing. | Synergistic effects slowing progression from acute to chronic. |
| Biomarker Development | Identifying markers for early mitochondrial distress. | Circulating mtDNA or ROS levels. | Specificity to kidney versus systemic. | Early intervention, preventing irreversible damage. |
This table maps future paths, drawing from ongoing research to advance care.
Acknowledgement
The creation of the article “Mitochondrial Redox Signaling and Oxidative Stress in Kidney Diseases” was made possible through the wealth of scientific knowledge available from reputable sources. The Examsmeta.com website expresses its gratitude to PubMed (pubmed.ncbi.nlm.nih.gov) for providing access to peer-reviewed studies that enriched our understanding of mitochondrial dynamics and redox signaling. Also, acknowledges ScienceDirect (www.sciencedirect.com) for its extensive database of research articles that informed the discussion on oxidative stress in kidney diseases.
Additionally, Nature Reviews (www.nature.com/nrneph) offered critical insights into the pathophysiology of acute and chronic kidney conditions. Their comprehensive reviews were instrumental in shaping the article’s depth and accuracy.

