The Tricarboxylic Acid (TCA) Cycle, often referred to as the Krebs Cycle or Citric Acid Cycle, stands at the core of how our cells generate energy. This intricate series of chemical reactions happens inside the mitochondria, those tiny powerhouses within our cells, and it’s essential for breaking down nutrients from the food we eat into usable energy. Imagine it as a bustling factory where carbohydrates, fats, and proteins are processed to fuel everything from muscle movements to brain functions. Without this cycle, life as we know it wouldn’t be possible, as it plays a pivotal role in aerobic respiration, the process that requires oxygen to produce ATP, the cell’s energy currency.
What makes the TCA cycle so fascinating is its efficiency and versatility. It doesn’t just churn out energy; it also provides building blocks for other vital molecules in the body, like amino acids and nucleotides. Discovered in the mid-20th century, this pathway has been studied extensively, revealing its connections to various metabolic processes. In simple terms, it takes the products from earlier stages of digestion, like pyruvate from glucose breakdown, and oxidizes them completely, releasing carbon dioxide and capturing energy in the form of electron carriers. These carriers then feed into the electron transport chain to make even more ATP.
Table of Contents
For anyone interested in biology, nutrition, or even health conditions like diabetes, understanding this cycle opens up a world of insights into how our bodies work on a molecular level.
Historical Background of the TCA Cycle
The story of the TCA cycle begins with a brilliant biochemist named Hans Krebs, who pieced together this puzzle in 1937 while working with pigeon flight muscles. He noticed that certain acids, like citric acid, were involved in the oxidation of carbohydrates, leading him to propose a cyclic pathway where molecules are regenerated at the end. This was a groundbreaking idea at the time, shifting the view from linear reactions to a looped system that maximizes efficiency. Krebs’ work built on earlier discoveries, such as those by Albert Szent-Györgyi on dicarboxylic acids, and it earned him the Nobel Prize in Physiology or Medicine in 1953, shared with Fritz Lipmann for his contributions to coenzyme A.
Before Krebs, scientists knew that cells respired aerobically, but the exact mechanisms were murky. Experiments with minced tissues and inhibitors helped map out the intermediates. For instance, adding malonate, which blocks succinate dehydrogenase, caused an accumulation of succinate, hinting at the sequence. This historical context shows how scientific progress often comes from incremental observations and clever experiments. Today, the cycle bears Krebs’ name as a tribute, and it’s taught in classrooms worldwide as a fundamental concept in biochemistry. Interestingly, similar pathways exist in bacteria and plants, suggesting it’s an ancient evolutionary adaptation for energy extraction.
Advancements in technology, like isotopic labeling in the 1940s, confirmed the cycle’s steps by tracking carbon atoms through the reactions. This not only validated Krebs’ model but also highlighted its role beyond energy production, such as in biosynthesis. The history underscores the importance of curiosity-driven research, as understanding this cycle has implications for medicine, agriculture, and even biofuel production.
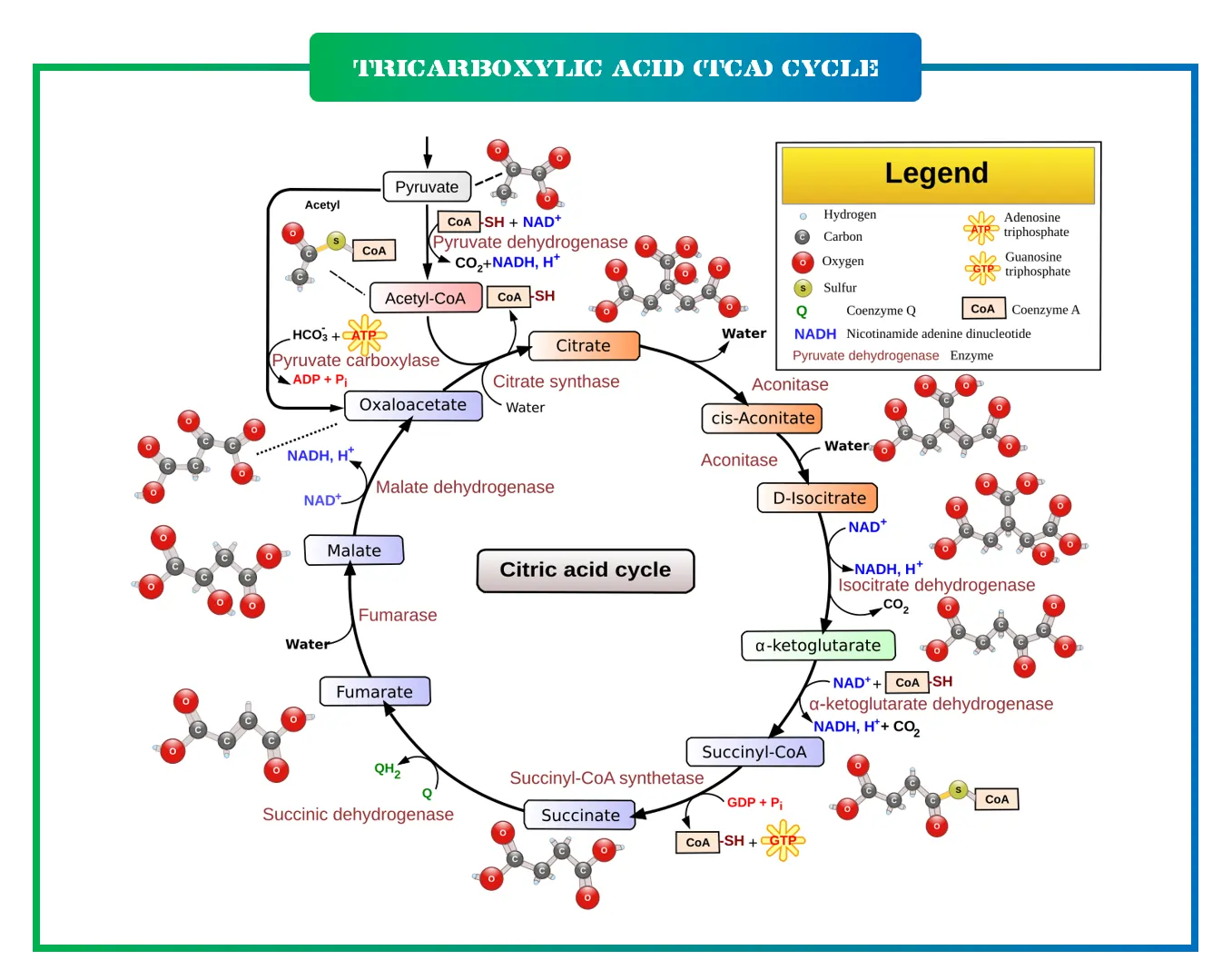
The Steps of the TCA Cycle: A Detailed Breakdown
The TCA cycle consists of eight enzymatic steps, forming a closed loop where the final product regenerates the starting molecule. It starts with acetyl-CoA, derived from pyruvate or fatty acids, combining with oxaloacetate to form citrate. Each turn of the cycle processes one acetyl group, releasing two carbon dioxide molecules and generating high-energy carriers like NADH and FADH2.
Let’s break it down step by step for clarity:
- Step 1: Formation of Citrate. Here, acetyl-CoA (a two-carbon molecule) joins with oxaloacetate (a four-carbon molecule) in a condensation reaction catalyzed by citrate synthase. This produces citrate, a six-carbon compound, and releases coenzyme A. It’s like merging two puzzle pieces to start the assembly line.
- Step 2: Isomerization to Isocitrate. Citrate is converted to isocitrate through an intermediate called aconitate. The enzyme aconitase facilitates this by first dehydrating citrate to cis-aconitate and then hydrating it to isocitrate. This step rearranges the molecule for the upcoming oxidations.
- Step 3: First Oxidative Decarboxylation. Isocitrate dehydrogenase oxidizes isocitrate to alpha-ketoglutarate, releasing CO2 and producing NADH from NAD+. This is a key energy-capturing point, and the enzyme requires magnesium or manganese ions.
- Step 4: Second Oxidative Decarboxylation. Alpha-ketoglutarate is further oxidized by the alpha-ketoglutarate dehydrogenase complex, similar to the pyruvate dehydrogenase complex. It yields succinyl-CoA, another CO2, and more NADH. This step involves multiple cofactors like thiamine pyrophosphate and lipoic acid.
- Step 5: Substrate-Level Phosphorylation. Succinyl-CoA synthetase converts succinyl-CoA to succinate, generating GTP (which can convert to ATP) and releasing coenzyme A. This is one of the few steps producing ATP directly in the cycle.
- Step 6: Oxidation of Succinate. Succinate dehydrogenase, embedded in the mitochondrial membrane, oxidizes succinate to fumarate, producing FADH2. This enzyme is unique as it’s part of both the TCA cycle and the electron transport chain.
- Step 7: Hydration to Malate. Fumarase adds water to fumarate, forming malate. This reversible reaction ensures the cycle flows smoothly.
- Step 8: Regeneration of Oxaloacetate. Finally, malate dehydrogenase oxidizes malate to oxaloacetate, producing another NADH. This closes the loop, ready for the next acetyl-CoA.
These steps highlight the cycle’s elegance, where each reaction sets up the next. For example, in muscle cells during exercise, the cycle ramps up to meet energy demands, processing more glucose-derived acetyl-CoA.
To visualize the energy output, consider that one glucose molecule leads to two turns of the cycle (since glycolysis produces two pyruvates). Each turn yields 3 NADH, 1 FADH2, and 1 GTP/ATP, so overall, it’s a significant contributor to the 30-32 ATP from complete glucose oxidation.
Key Enzymes and Intermediates in the TCA Cycle
The enzymes driving the TCA cycle are highly specific proteins that lower activation energies for reactions. Citrate synthase, for instance, is allosterically regulated and sets the pace. Intermediates like citrate, isocitrate, alpha-ketoglutarate, succinyl-CoA, succinate, fumarate, malate, and oxaloacetate are not just transitory; they serve as precursors for other biomolecules.
Here’s a comprehensive table outlining the enzymes, their substrates, products, and cofactors:
| Step Number | Enzyme Name | Substrate | Product | Cofactors Involved | Reaction Type |
|---|---|---|---|---|---|
| 1 | Citrate Synthase | Acetyl-CoA + Oxaloacetate | Citrate + CoA | None | Condensation |
| 2 | Aconitase | Citrate | Isocitrate (via cis-Aconitate) | Fe-S clusters | Isomerization |
| 3 | Isocitrate Dehydrogenase | Isocitrate + NAD+ | Alpha-Ketoglutarate + CO2 + NADH | Mg2+ or Mn2+, NAD+ | Oxidative Decarboxylation |
| 4 | Alpha-Ketoglutarate Dehydrogenase Complex | Alpha-Ketoglutarate + NAD+ + CoA | Succinyl-CoA + CO2 + NADH | TPP, Lipoic Acid, FAD, NAD+, CoA | Oxidative Decarboxylation |
| 5 | Succinyl-CoA Synthetase | Succinyl-CoA + GDP + Pi | Succinate + GTP + CoA | Mg2+, GDP | Substrate-Level Phosphorylation |
| 6 | Succinate Dehydrogenase | Succinate + FAD | Fumarate + FADH2 | FAD, Fe-S clusters | Oxidation |
| 7 | Fumarase | Fumarate + H2O | Malate | None | Hydration |
| 8 | Malate Dehydrogenase | Malate + NAD+ | Oxaloacetate + NADH | NAD+ | Oxidation |
This table shows the diversity of reactions, from oxidations to hydrations. Intermediates can exit the cycle for other uses; for example, alpha-ketoglutarate is a precursor for glutamate, an amino acid crucial for neurotransmission. In plants, succinate links to chlorophyll synthesis, illustrating the cycle’s broader role.
Enzymes are located in the mitochondrial matrix, except for succinate dehydrogenase on the inner membrane. Mutations in these enzymes can lead to rare diseases, emphasizing their criticality.
Regulation of the TCA Cycle: Keeping Balance in Metabolism
The TCA cycle isn’t always running at full speed; it’s tightly regulated to match cellular needs. Regulation occurs mainly at three points: isocitrate dehydrogenase, alpha-ketoglutarate dehydrogenase, and citrate synthase. These enzymes respond to energy levels through allosteric effectors and covalent modifications.
For instance, high ATP or NADH levels inhibit isocitrate dehydrogenase, slowing the cycle when energy is abundant. Conversely, ADP and calcium ions activate it during high demand, like in contracting muscles. Alpha-ketoglutarate dehydrogenase is similarly inhibited by succinyl-CoA and NADH, preventing overload.
Hormonal influences, such as insulin promoting anaplerotic reactions to replenish intermediates, add another layer. Anaplerosis, meaning “filling up,” involves reactions like pyruvate carboxylase converting pyruvate to oxaloacetate when levels drop.
In fasting states, the cycle shifts to support gluconeogenesis, using intermediates like malate. This adaptability ensures survival under varying conditions.
Consider a real-world example: During intense exercise, lactate from anaerobic glycolysis enters the cycle via conversion to pyruvate, boosting energy production. In contrast, in well-fed states, excess acetyl-CoA from carbs diverts to fat synthesis.
A detailed table on regulatory mechanisms:
| Enzyme | Positive Regulators (Activators) | Negative Regulators (Inhibitors) | Mechanism | Physiological Context |
|---|---|---|---|---|
| Citrate Synthase | None (mainly substrate availability) | ATP, NADH, Succinyl-CoA | Allosteric Inhibition | High energy slows entry |
| Isocitrate Dehydrogenase | ADP, Ca2+, AMP | ATP, NADH | Allosteric Activation/Inhibition | Energy demand boosts, surplus slows |
| Alpha-Ketoglutarate Dehydrogenase | Ca2+ | Succinyl-CoA, NADH, ATP | Allosteric and Product Inhibition | Prevents intermediate buildup |
| Pyruvate Dehydrogenase (entry to cycle) | Insulin, Low Acetyl-CoA/CoA ratio | Acetyl-CoA, NADH, Phosphorylation by kinase | Covalent Modification | Fed state activates, fasting inhibits |
| Succinate Dehydrogenase | None specific | Oxaloacetate | Competitive Inhibition | Links to electron transport |
This regulation prevents wasteful energy use and maintains homeostasis.
The Importance of the TCA Cycle in Cellular Metabolism
Beyond energy, the TCA cycle is amphibolic, meaning it serves both catabolic (breakdown) and anabolic (buildup) roles. It integrates metabolism by accepting inputs from glycolysis, beta-oxidation of fats, and amino acid degradation. For energy, it produces reducing equivalents (NADH, FADH2) that yield about 10 ATP per turn via oxidative phosphorylation.
In biosynthesis, citrate exports to the cytosol for fatty acid synthesis, crucial for cell membranes. Oxaloacetate and malate support gluconeogenesis in the liver, maintaining blood sugar. In the brain, alpha-ketoglutarate converts to glutamate, a key neurotransmitter.
In immune cells, the cycle adapts during inflammation; macrophages break it to accumulate itaconate, an antimicrobial compound. In cancer, mutations in enzymes like isocitrate dehydrogenase lead to oncometabolites that promote tumor growth, highlighting its role in disease.
Plants use it for carbon fixation in roots, and bacteria for diverse metabolisms. This universality underscores its evolutionary conservation.
For a nutritional angle, vitamins like B1 (thiamine) are cofactors; deficiencies cause beriberi, disrupting the cycle and leading to fatigue.
Connections to Other Metabolic Pathways
The TCA cycle doesn’t operate in isolation; it’s linked to glycolysis via pyruvate dehydrogenase, converting pyruvate to acetyl-CoA. Fatty acids enter through beta-oxidation, producing acetyl-CoA. Amino acids like alanine feed in at various points.
The urea cycle intersects at fumarate, recycling carbons. The electron transport chain receives electrons from NADH/FADH2, coupling the cycle to ATP synthesis.
An example: In diabetes, high blood sugar overwhelms the cycle, leading to ketone bodies from excess acetyl-CoA. In starvation, it processes fats efficiently.
A table mapping connections:
| Linked Pathway | Entry/Exit Point in TCA | Molecule Involved | Purpose |
|---|---|---|---|
| Glycolysis | Acetyl-CoA entry | Pyruvate → Acetyl-CoA | Carbohydrate oxidation |
| Beta-Oxidation | Acetyl-CoA entry | Fatty acyl-CoA → Acetyl-CoA | Fat breakdown |
| Amino Acid Catabolism | Various (e.g., alpha-ketoglutarate) | Glutamate → Alpha-Ketoglutarate | Protein degradation |
| Gluconeogenesis | Oxaloacetate/Malate exit | Oxaloacetate → Phosphoenolpyruvate | Glucose synthesis |
| Fatty Acid Synthesis | Citrate exit | Citrate → Acetyl-CoA (cytosolic) | Lipid buildup |
| Urea Cycle | Fumarate exchange | Fumarate ↔ Aspartate | Nitrogen waste removal |
| Pentose Phosphate Pathway | Indirect via NADPH | NADPH for reductions | Reductive biosynthesis |
These links make the cycle a metabolic crossroads.
Disorders Associated with the TCA Cycle
Genetic defects in TCA enzymes cause rare mitochondrial disorders. For example, succinate dehydrogenase mutations lead to paragangliomas, tumors due to succinate accumulation mimicking hypoxia.
Fumarase deficiency causes fumaric aciduria, with neurological issues from acid buildup. Alpha-ketoglutarate dehydrogenase defects contribute to lactic acidosis.
In cancer, IDH1/2 mutations produce 2-hydroxyglutarate, an oncometabolite altering epigenetics. Therapies target these, like IDH inhibitors.
Environmental factors, like toxins inhibiting enzymes (e.g., fluoroacetate blocking aconitase), mimic disorders.
Understanding these helps in diagnosing and treating metabolic diseases.
Real-World Examples and Applications of the TCA Cycle
In sports nutrition, athletes consume carbs to fuel the cycle for endurance. In winemaking, yeast’s cycle affects flavor compounds.
In medicine, PET scans use cycle intermediates for imaging. In biotech, engineering microbes enhances cycle for biofuel production.
Consider a diabetic patient: Impaired regulation leads to complications, but metformin activates AMPK, indirectly boosting cycle efficiency.
In agriculture, optimizing plant cycles improves crop yields under stress.
The cycle’s study continues, with recent insights into its role in aging and immunity, promising future therapies.
This comprehensive look at the TCA cycle reveals its profound impact on life, from microscopic reactions to whole-body health. Exploring it deepens appreciation for biology’s complexity.
Frequently Asked Questions
FAQ 1: What Are the Detailed Steps of the TCA Cycle?
The TCA cycle, also known as the Krebs cycle, is a series of eight enzymatic reactions that occur in the mitochondrial matrix of eukaryotic cells. It begins with the entry of acetyl-CoA, which is typically derived from the breakdown of carbohydrates, fats, or proteins. Each step transforms intermediates while releasing carbon dioxide and generating energy-rich molecules like NADH and FADH2. These steps are crucial for efficient energy extraction, and understanding them helps explain how cells maintain their metabolic balance.
To provide a clear overview, here’s a well-structured table detailing each step, including the enzymes, substrates, products, and key reaction equations in LaTeX format for precision:
| Step | Enzyme | Substrate | Product | Reaction Equation | Notes |
|---|---|---|---|---|---|
| 1 | Citrate Synthase | Acetyl-CoA + Oxaloacetate + H₂O | Citrate + CoA-SH | Acetyl-CoA + Oxaloacetate + H2O → Citrate + CoA-SH | This condensation reaction initiates the cycle and is irreversible, regulated by substrate availability. |
| 2 | Aconitase | Citrate | cis-Aconitate + H₂O (intermediate), then Isocitrate | Citrate ⇌[Aconitase] cis-Aconitate + H2O ⇌[Aconitase] Isocitrate | Involves dehydration followed by hydration, making isocitrate ready for oxidation. |
| 3 | Isocitrate Dehydrogenase | Isocitrate + NAD⁺ | α-Ketoglutarate + CO₂ + NADH + H⁺ | Isocitrate + NAD+ → α-Ketoglutarate + CO2 + NADH + H+ | First oxidative decarboxylation, producing NADH and releasing CO₂; requires Mg²⁺ or Mn²⁺. |
| 4 | α-Ketoglutarate Dehydrogenase Complex | α-Ketoglutarate + NAD⁺ + CoA-SH | Succinyl-CoA + CO₂ + NADH + H⁺ | α-Ketoglutarate + NAD+ + CoA-SH → Succinyl-CoA + CO2 + NADH + H+ | Multi-enzyme complex similar to pyruvate dehydrogenase; uses cofactors like TPP and FAD. |
| 5 | Succinyl-CoA Synthetase | Succinyl-CoA + GDP + Pᵢ | Succinate + GTP + CoA-SH | Succinyl-CoA + GDP + Pi → Succinate + GTP + CoA-SH | Substrate-level phosphorylation, generating GTP which can be converted to ATP. |
| 6 | Succinate Dehydrogenase | Succinate + FAD | Fumarate + FADH₂ | Succinate + FAD → Fumarate + FADH2 | Oxidation step linked to the electron transport chain; enzyme is membrane-bound. |
| 7 | Fumarase | Fumarate + H₂O | Malate | Fumarate + H2O ⇌ Malate | Hydration reaction, reversible and stereospecific. |
| 8 | Malate Dehydrogenase | Malate + NAD⁺ | Oxaloacetate + NADH + H⁺ | Malate + NAD+ → Oxaloacetate + NADH + H+ | Final oxidation, regenerating oxaloacetate to close the cycle. |
This table illustrates the cycle’s efficiency, where each turn processes one acetyl-CoA unit, releasing two CO₂ molecules and producing three NADH, one FADH₂, and one GTP/ATP. In practice, these steps integrate with cellular demands, such as during exercise when the cycle accelerates to meet energy needs.
FAQ 2: How Is the TCA Cycle Regulated to Match Cellular Energy Needs?
Regulation of the TCA cycle ensures that cells produce energy only when necessary, preventing waste and maintaining metabolic harmony. Primarily, this control happens at key enzymatic steps through allosteric mechanisms, where molecules bind to enzymes and alter their activity. For example, high levels of ATP and NADH act as signals of energy abundance, inhibiting enzymes like isocitrate dehydrogenase and α-ketoglutarate dehydrogenase to slow the cycle. Conversely, when energy is low, ADP and AMP activate these enzymes, ramping up production.
Beyond allosteric regulation, substrate availability plays a crucial role. The cycle’s entry point, citrate synthase, depends on the concentrations of acetyl-CoA and oxaloacetate; if either is scarce, the cycle slows. Post-translational modifications, such as phosphorylation, also fine-tune enzyme function—insulin, for instance, dephosphorylates pyruvate dehydrogenase to promote acetyl-CoA entry in fed states. Calcium ions, released during muscle contraction, activate dehydrogenases, linking the cycle to physical activity.
Hormonal influences add another layer, with glucagon in fasting states favoring gluconeogenesis, which draws intermediates from the cycle. Anaplerotic reactions replenish these lost intermediates, ensuring continuity. Overall, this multifaceted regulation makes the TCA cycle responsive to the body’s dynamic needs, from rest to intense exertion. In diseases like diabetes, disrupted regulation can lead to metabolic imbalances, highlighting its clinical importance.
FAQ 3: What Role Does the TCA Cycle Play in Cancer Development and Progression?
The TCA cycle’s involvement in cancer stems from its central position in metabolism, where alterations can fuel uncontrolled cell growth. In healthy cells, the cycle efficiently oxidizes nutrients for energy, but cancer cells often reprogram it to support rapid proliferation. Mutations in enzymes like isocitrate dehydrogenase lead to the production of oncometabolites, such as 2-hydroxyglutarate, which disrupt gene expression and promote tumor formation.
Beyond mutations, cancer cells may truncate the cycle, accumulating intermediates like succinate or fumarate that stabilize hypoxia-inducible factors, mimicking low-oxygen conditions and enhancing angiogenesis. This metabolic shift, part of the Warburg effect, prioritizes biosynthesis over full oxidation, even in oxygen-rich environments.
Key insights into TCA cycle’s role in cancer include:
- Oncometabolite Accumulation: Defective enzymes cause buildup of molecules that alter epigenetics, increasing cancer risk in tissues like the brain or kidneys.
- Therapeutic Targets: Inhibitors targeting mutant IDH enzymes have shown promise in treating gliomas and leukemias by reducing oncometabolite levels.
- Immune System Interactions: Altered cycle metabolites can suppress immune responses, allowing tumors to evade detection; recent studies explore boosting anaplerosis to enhance immunotherapy.
- Prognostic Indicators: Higher TCA activity in certain cancers correlates with poorer outcomes, as seen in lung cancer where glucose heavily contributes to cycle intermediates.
Understanding these mechanisms opens doors to novel treatments, emphasizing the cycle’s dual role in energy and signaling.
FAQ 4: How Does the TCA Cycle Connect to Glycolysis and Other Metabolic Pathways?
The TCA cycle serves as a metabolic hub, integrating inputs from various pathways to optimize energy and biosynthesis. Its connections ensure seamless nutrient processing across the cell.
Here’s a detailed table outlining major linkages, including entry/exit points and purposes:
| Connected Pathway | Entry/Exit Point | Key Molecule | Purpose | Example in Physiology |
|---|---|---|---|---|
| Glycolysis | Entry via Acetyl-CoA | Pyruvate → Acetyl-CoA | Oxidizes glucose-derived pyruvate for energy | During aerobic exercise, boosts ATP production. |
| Beta-Oxidation | Entry via Acetyl-CoA | Fatty acids → Acetyl-CoA | Breaks down fats for fuel | In fasting, provides energy from stored lipids. |
| Amino Acid Catabolism | Various intermediates | Glutamate → α-Ketoglutarate | Degrades proteins into cycle fuels | Supports energy during starvation or muscle breakdown. |
| Gluconeogenesis | Exit via Oxaloacetate/Malate | Oxaloacetate → PEP | Synthesizes glucose | Maintains blood sugar in liver during low-carb states. |
| Fatty Acid Synthesis | Exit via Citrate | Citrate → Cytosolic Acetyl-CoA | Builds lipids | In fed states, stores excess energy as fat. |
| Urea Cycle | Exchange at Fumarate | Fumarate ↔ Aspartate | Removes nitrogen waste | Detoxifies ammonia in liver cells. |
| Pentose Phosphate Pathway | Indirect via NADPH | NADPH for reductions | Supports biosynthesis | Provides reducing power for antioxidant defense. |
These interconnections highlight the cycle’s versatility, allowing adaptation to nutritional changes.
FAQ 5: What Is the Total Energy Yield from the Krebs Cycle per Glucose Molecule?
Calculating the energy yield from the Krebs cycle reveals its efficiency in cellular respiration. For one glucose molecule, glycolysis produces two pyruvates, each converted to acetyl-CoA, fueling two turns of the cycle. Each turn generates 3 NADH, 1 FADH₂, and 1 GTP (equivalent to ATP), so per glucose, that’s 6 NADH, 2 FADH₂, and 2 ATP from the cycle itself.
However, the true yield comes from oxidative phosphorylation, where NADH and FADH₂ donate electrons to the electron transport chain. Each NADH typically yields about 2.5 ATP, and each FADH₂ about 1.5 ATP. Thus, the 6 NADH produce around 15 ATP, and 2 FADH₂ produce 3 ATP, totaling 20 ATP from the cycle’s carriers, plus the 2 direct ATP.
Including glycolysis (2 ATP net) and pyruvate oxidation (2 NADH → 5 ATP), complete glucose oxidation yields approximately 30-32 ATP. This varies slightly due to shuttle systems transporting reducing equivalents into mitochondria. In anaerobic conditions, the cycle halts, limiting yield to glycolysis’ 2 ATP.
FAQ 6: What Are the Key Differences in the TCA Cycle Between Prokaryotes and Eukaryotes?
In prokaryotes, like bacteria, the TCA cycle occurs in the cytoplasm since they lack mitochondria, allowing direct integration with other cytosolic processes. This setup enables faster responses to environmental changes, such as nutrient shifts, but exposes the cycle to broader cellular fluctuations.
Eukaryotes, however, confine the cycle to the mitochondrial matrix, compartmentalizing it for efficiency and protection. This requires transport systems for metabolites, like the malate-aspartate shuttle for NADH equivalents. Enzymatic differences exist too; for instance, isocitrate dehydrogenase in eukaryotes uses NAD⁺ primarily, while some prokaryotes employ NADP⁺, linking to biosynthetic pathways.
Evolutionarily, the eukaryotic cycle likely arose from endosymbiotic prokaryotes, explaining similarities, but eukaryotes have additional regulatory layers, like calcium signaling, absent in most prokaryotes. These distinctions impact applications, from antibiotic design targeting bacterial cycles to understanding mitochondrial diseases in humans.
FAQ 7: Who Discovered the Citric Acid Cycle and What Was the Historical Context?
The discovery of the citric acid cycle traces back to the 1930s, amid efforts to unravel cellular respiration. Hans Adolf Krebs, a German-born biochemist fleeing Nazi persecution, pieced it together in 1937 at the University of Sheffield. Building on earlier work by Albert Szent-Györgyi on dicarboxylic acids and Martius and Knoop on citrate’s role, Krebs observed that citrate catalyzed carbohydrate oxidation in muscle tissues.
His pivotal experiment with pigeon breast muscle showed citrate regenerating oxaloacetate, forming a cyclic pathway. Initially rejected by Nature, his findings were published in Enzymologia, earning him the 1953 Nobel Prize. This breakthrough shifted biochemistry from linear to cyclic models, influencing fields like medicine and agriculture.
Post-discovery, isotopic tracing in the 1940s confirmed the steps, revealing the cycle’s universality across life forms.
FAQ 8: What Are Anaplerotic Reactions and Why Are They Important in the TCA Cycle?
Anaplerotic reactions replenish TCA cycle intermediates depleted during biosynthesis, ensuring the cycle’s continuity. Without them, the cycle would stall as intermediates like α-ketoglutarate are diverted for amino acid synthesis.
Key anaplerotic pathways include:
- Pyruvate Carboxylase Reaction: Converts pyruvate to oxaloacetate using biotin and ATP, vital in liver for gluconeogenesis.
- Glutamate Dehydrogenase: Forms α-ketoglutarate from glutamate, linking nitrogen metabolism.
- Propionyl-CoA Carboxylase: Produces succinyl-CoA from odd-chain fatty acids or certain amino acids.
These reactions balance cataplerosis, the exit of intermediates, maintaining metabolic flexibility in varying conditions like fasting or growth.
FAQ 9: How Does the TCA Cycle Support Biosynthetic Processes in Cells?
The amphibolic nature of the TCA cycle allows it to catabolize fuels while providing precursors for synthesis. Citrate, for example, exits to the cytosol for fatty acid production via ATP-citrate lyase.
A table of biosynthetic roles:
| Intermediate | Biosynthetic Product | Pathway Involved | Cellular Importance |
|---|---|---|---|
| Citrate | Fatty Acids/Cholesterol | Exported to cytosol | Membrane and hormone synthesis. |
| α-Ketoglutarate | Glutamate/Amino Acids | Transamination | Neurotransmitters and proteins. |
| Succinyl-CoA | Heme/Porphyrins | Heme biosynthesis | Oxygen transport in blood. |
| Oxaloacetate | Aspartate/Nucleotides | Aminotransferases | DNA/RNA building blocks. |
| Malate | Glucose (via gluconeogenesis) | Malate shuttle | Blood sugar regulation. |
This dual function underscores its role in growth and repair.
FAQ 10: Why Is the TCA Cycle Considered Amphibolic and What Does That Mean for Metabolism?
Amphibolic means the TCA cycle serves both catabolic and anabolic purposes, breaking down molecules for energy while building others. Catabolically, it oxidizes acetyl-CoA to CO₂, capturing energy in NADH/FADH₂ for ATP synthesis. Anabolically, intermediates fuel synthesis of lipids, amino acids, and nucleotides, essential for cell proliferation.
This duality allows metabolic flexibility; in growing tissues, more intermediates are withdrawn, requiring anaplerosis to refill. In energy-demanding states, full oxidation predominates. Disruptions, as in cancer, favor anabolism, promoting tumor growth. Overall, its amphibolic character integrates metabolism, adapting to nutritional and physiological demands.
FAQ 11: How Does the TCA Cycle Function in Plant Metabolism?
The TCA cycle in plants operates similarly to that in animals, serving as a central hub for energy production and biosynthesis, but with unique adaptations suited to photosynthetic organisms. In plant cells, the cycle occurs in mitochondria, where it oxidizes acetyl-CoA derived from various sources, including pyruvate from glycolysis or fatty acids from lipid breakdown. However, plants also integrate the cycle with chloroplast activities, allowing for efficient carbon assimilation during daylight. For instance, during photosynthesis, the cycle provides carbon skeletons for amino acid synthesis while minimizing CO2 release to avoid competing with carbon fixation.
Unlike in animals, where the cycle primarily focuses on ATP generation, in plants it plays a dual role in supporting growth under varying light conditions. In the dark, it ramps up to provide energy from stored reserves, while in light, it shifts toward anaplerotic functions, replenishing intermediates for nitrogen assimilation. Environmental stresses like drought or salinity can alter cycle flux, often leading to accumulation of intermediates that act as osmoprotectants or signaling molecules. This flexibility highlights the cycle’s evolutionary tuning to plant lifestyles.
To detail the specific enzymes and their plant-specific roles, here’s a comprehensive table:
| Enzyme | Role in Plants | Substrate/Product | Unique Plant Adaptation | Physiological Impact |
|---|---|---|---|---|
| Citrate Synthase | Initiates cycle by forming citrate | Acetyl-CoA + Oxaloacetate → Citrate | Regulated by light via redox signals | Supports root exudation of citrate for nutrient uptake. |
| Aconitase | Converts citrate to isocitrate | Citrate → Isocitrate | Sensitive to oxidative stress; isoforms in cytosol and mitochondria | Involved in iron homeostasis and stress responses. |
| Isocitrate Dehydrogenase | Oxidizes isocitrate, producing NADH | Isocitrate → α-Ketoglutarate + CO2 + NADH | NADP-dependent isoform links to Calvin cycle | Provides NADPH for antioxidant defense in leaves. |
| α-Ketoglutarate Dehydrogenase | Decarboxylates α-ketoglutarate | α-Ketoglutarate → Succinyl-CoA + CO2 + NADH | Thioredoxin-regulated for day-night shifts | Crucial for glutamate synthesis in nitrogen metabolism. |
| Succinyl-CoA Synthetase | Generates succinate and GTP | Succinyl-CoA → Succinate + GTP | Coupled to photorespiration | Aids in energy transfer during high metabolic demand. |
| Succinate Dehydrogenase | Oxidizes succinate to fumarate | Succinate → Fumarate + FADH2 | Part of complex II in electron transport | Enhances respiration under hypoxia in roots. |
| Fumarase | Hydrates fumarate to malate | Fumarate → Malate | Cytosolic isoform for malate shuttling | Facilitates organic acid accumulation in fruits. |
| Malate Dehydrogenase | Regenerates oxaloacetate | Malate → Oxaloacetate + NADH | Multiple isoforms for C4/CAM photosynthesis | Key in malate valve for balancing redox states. |
This table underscores how plant TCA enzymes are modulated by environmental cues, ensuring survival and productivity.
FAQ 12: What Happens to the TCA Cycle During Exercise?
During physical activity, the TCA cycle accelerates dramatically to meet the body’s surging energy demands, acting as a metabolic accelerator that processes fuels more efficiently. As muscles contract, the cycle ramps up flux through its intermediates, drawing in more acetyl-CoA from carbohydrate and fat breakdown. This increase is driven by rising ADP and calcium levels, which activate key enzymes like isocitrate dehydrogenase, ensuring a steady supply of NADH and FADH2 for the electron transport chain. In moderate exercise, the cycle handles the load aerobically, but in intense bouts, it may accumulate intermediates like succinate, signaling for enhanced mitochondrial biogenesis over time.
Adaptations from regular exercise further optimize the cycle; trained individuals show expanded TCA intermediate pools, allowing for greater endurance without rapid fatigue. For example, endurance training boosts enzyme expression, leading to a 70-fold flux increase during submaximal efforts compared to rest. This not only sustains ATP production but also mitigates lactate buildup by integrating with glycolysis.
In recovery phases post-exercise, the cycle slows but remains active, aiding in replenishing glycogen stores and clearing metabolic byproducts. Disruptions, such as in untrained people, can lead to quicker exhaustion due to mismatched pyruvate supply and cycle capacity. Overall, the TCA cycle’s responsiveness during exercise exemplifies its role in human performance and health.
FAQ 13: Which Vitamins and Cofactors Are Essential for the TCA Cycle?
Vitamins and cofactors are indispensable for the smooth operation of the TCA cycle, acting as molecular assistants that enable enzymes to perform their tasks efficiently. Without them, the cycle would grind to a halt, impairing energy production and leading to health issues like fatigue or neurological problems. For instance, several B vitamins serve as precursors for coenzymes that facilitate oxidation and decarboxylation steps, ensuring the cycle’s intermediates are properly transformed.
Key vitamins and their roles include:
- Thiamine (Vitamin B1): Forms thiamine pyrophosphate, crucial for α-ketoglutarate dehydrogenase in decarboxylating α-ketoglutarate; deficiency causes beriberi, disrupting energy metabolism.
- Riboflavin (Vitamin B2): Precursor to FAD, used by succinate dehydrogenase to oxidize succinate; supports FADH2 production for the electron chain.
- Niacin (Vitamin B3): Generates NAD+, essential for multiple dehydrogenases like malate dehydrogenase; pellagra from deficiency affects skin and nerves due to poor NADH yield.
- Pantothenic Acid (Vitamin B5): Component of coenzyme A, vital for acetyl-CoA entry and succinyl-CoA formation; ensures substrate delivery.
- Biotin (Vitamin B7): Involved in anaplerotic reactions via pyruvate carboxylase, replenishing oxaloacetate; aids cycle maintenance during high demand.
These elements highlight how dietary intake directly influences cellular energy pathways.
FAQ 14: What Are Common Inhibitors and Poisons That Affect the TCA Cycle?
Inhibitors and poisons targeting the TCA cycle can severely disrupt cellular energy production, often leading to toxicity or disease by halting key reactions. These compounds interfere at specific enzymatic steps, either competitively blocking substrates or irreversibly damaging enzymes. Understanding them is crucial for toxicology and medicine, as they reveal vulnerabilities in metabolism.
Here’s a detailed table of notable inhibitors, their targets, mechanisms, and effects:
| Inhibitor/Poison | Target Enzyme | Mechanism | Physiological Effects | Examples/Sources |
|---|---|---|---|---|
| Fluoroacetate | Aconitase | Converts to fluorocitrate, which binds tightly, preventing citrate conversion | Causes convulsions and heart failure; used in rodenticides. | |
| Malonate | Succinate Dehydrogenase | Competitive inhibition, mimicking succinate | Reduces FADH2 production, leading to energy deficit in tissues. | |
| Arsenite | Pyruvate Dehydrogenase/α-Ketoglutarate Dehydrogenase | Binds to lipoic acid cofactor, blocking decarboxylation | Arsenic poisoning symptoms like nausea; inhibits acetyl-CoA entry. | |
| Cyanide | Electron Transport Chain (Indirect TCA Impact) | Inhibits cytochrome c oxidase, backing up TCA intermediates | Rapid toxicity, halting respiration; affects cycle flux indirectly. | |
| Rotenone | Complex I (NADH Dehydrogenase) | Prevents NADH oxidation, slowing TCA | Used as pesticide; causes Parkinson’s-like symptoms from mitochondrial stress. |
This overview shows how such agents can be both environmental hazards and tools for studying metabolism.
FAQ 15: What Are the Evolutionary Origins of the Citric Acid Cycle?
The citric acid cycle’s evolutionary roots trace back to ancient microbial life, likely emerging as a primitive pathway for carbon fixation and energy extraction in pre-oxygen environments. Initially, it may have functioned in reverse as a reductive cycle, using CO2 to build organic molecules, a process still seen in some autotrophic bacteria today. Over time, as oxygen levels rose, it adapted into the oxidative form we know, efficiently breaking down nutrients while conserving energy.
This transition reflects modular evolution, where the cycle pieced together from simpler reaction sequences for amino acid biosynthesis. Genomic studies across prokaryotes and eukaryotes show variations, suggesting precursors in early life forms that combined linear pathways into a loop for efficiency.
In eukaryotes, the cycle’s mitochondrial localization points to endosymbiotic origins from bacterial ancestors, with gene transfers to the nucleus enhancing regulation. Recent discoveries of abiotic synthesis of intermediates under early Earth conditions support its prebiotic feasibility, bridging chemistry to biology.
FAQ 16: How Does the TCA Cycle Influence the Immune System?
The TCA cycle extends beyond energy production to profoundly shape immune responses, with its metabolites acting as signaling molecules that modulate inflammation and pathogen defense. In macrophages, for example, cycle rewiring during activation leads to itaconate accumulation, which inhibits bacterial growth and tempers excessive inflammation. This metabolic shift supports the transition from resting to pro-inflammatory states, ensuring effective immunity.
Key influences include:
- Succinate Accumulation: Stabilizes hypoxia-inducible factors, promoting cytokine production like IL-1β for acute responses.
- Citrate Export: Fuels fatty acid synthesis for membrane expansion in proliferating immune cells.
- α-Ketoglutarate Levels: Regulates epigenetic modifications, influencing T-cell differentiation and tolerance.
- Fumarate Effects: Inhibits certain enzymes, altering antiviral strategies in infected cells.
These roles position the cycle as a therapeutic target for autoimmune diseases and infections.
FAQ 17: How Is the TCA Cycle Disrupted in Diabetes?
In diabetes, the TCA cycle faces significant disruptions due to chronic hyperglycemia and insulin resistance, leading to impaired mitochondrial function and reduced energy efficiency. High glucose levels overwhelm the cycle, causing an influx of acetyl-CoA that isn’t fully processed, resulting in metabolite imbalances and oxidative stress. This often manifests as decreased flux through key steps, like reduced isocitrate dehydrogenase activity, which hampers NADH production and ATP synthesis.
Complications arise in tissues like the pancreas and nerves, where cycle intermediates decline, exacerbating neuropathy and beta-cell dysfunction. In type 2 diabetes, fatty acid overload further inhibits the cycle, promoting ketone accumulation. Therapeutic strategies, such as metformin, aim to restore balance by enhancing AMPK activity, indirectly boosting cycle efficiency.
Overall, these disruptions contribute to the metabolic chaos in diabetes, underscoring the need for interventions targeting mitochondrial health.
FAQ 18: What Are the Industrial and Biotechnological Applications of the TCA Cycle?
The TCA cycle’s versatility makes it a cornerstone in biotechnology, where engineers manipulate it in microbes to produce valuable compounds like organic acids and biofuels. By optimizing cycle flux, industries enhance yields of products used in food, pharmaceuticals, and materials.
A table of applications:
| Application | Microorganism/Method | Product | Benefits | Challenges |
|---|---|---|---|---|
| Citric Acid Production | Aspergillus niger fermentation | Citric Acid | Food additive, high yield via citrate synthase overexpression. | Nutrient optimization needed. |
| Succinic Acid Synthesis | Engineered E. coli with TCA rewiring | Succinic Acid | Bioplastics precursor; anaerobic conditions boost output. | |
| Glutamate Overproduction | Corynebacterium glutamicum | L-Glutamate | Flavor enhancer; α-ketoglutarate diversion. | |
| Biofuel Enhancement | Yeast strains with modified cycle | Ethanol/Butanol | Improved carbon efficiency; reduces CO2 loss. | Genetic stability issues. |
| Pharmaceutical Intermediates | Bacillus subtilis | Itaconic Acid | Polymer building block; cycle truncation for accumulation. |
These uses demonstrate the cycle’s industrial potential.
FAQ 19: What Are Common Misconceptions About the TCA Cycle?
A frequent misunderstanding is that the TCA cycle solely produces ATP, overlooking its vital role in biosynthesis; it generates precursors for amino acids and lipids, making it amphibolic rather than purely catabolic. Another myth is that it’s a rigid loop, but in reality, it adapts dynamically, with branches for anaplerosis and cataplerosis.
Common misconceptions include:
- It’s Only Mitochondrial: While primarily there, cytosolic isoforms exist for specific functions like in plants.
- Oxygen Is Directly Required: The cycle itself doesn’t use O2; it relies on the electron chain for regeneration of NAD+.
- All Intermediates Are Equal: Some, like succinate, have signaling roles beyond energy.
- It’s Inefficient in Cancer: Actually, tumors often enhance parts for growth advantages.
Clarifying these enhances biochemical literacy.
FAQ 20: What Are Future Research Directions in TCA Cycle Studies?
Future explorations of the TCA cycle promise to unravel its complexities in health and disease, focusing on metabolite signaling and therapeutic interventions. Researchers aim to map tissue-specific variations, using advanced metabolomics to understand how cycle dynamics influence aging and neurodegeneration.
Emphasis will be on integrating the cycle with epigenetics, where intermediates like α-ketoglutarate modulate gene expression, opening avenues for cancer treatments. In environmental contexts, studies on ocean worlds could reveal prebiotic analogs, informing origins of life.
Biotech advancements may engineer cycle-efficient microbes for sustainable production, while clinical trials target inhibitors for metabolic disorders. This multifaceted approach will deepen our grasp of life’s metabolic core.
Acknowledgement
The Examsmeta.com website expresses its humble gratitude to the invaluable resources that contributed to the development of the article “Tricarboxylic Acid (TCA) Cycle: The Heart of Cellular Energy Production.” The comprehensive insights provided by PubMed (pubmed.ncbi.nlm.nih.gov), ScienceDirect (www.sciencedirect.com), Nature (www.nature.com), and BiochemJ (www.biochemj.org) were instrumental in ensuring the scientific accuracy and depth of the content. These platforms offered access to peer-reviewed studies, detailed reviews, and cutting-edge research on the TCA cycle, enabling a thorough exploration of its mechanisms, regulation, and applications.
Key contributions from these sources include:
- PubMed: Provided extensive access to primary research articles on TCA cycle enzymes and their roles in disease, particularly cancer and metabolic disorders.
- ScienceDirect: Offered in-depth reviews on the cycle’s evolutionary origins and its adaptations in plants and prokaryotes.
- Nature: Contributed high-impact studies on the cycle’s role in immune responses and its therapeutic potential in biotechnology.
- BiochemJ: Supplied detailed biochemical analyses of enzyme regulation and cofactor requirements, enhancing the article’s technical precision.

