Post-translational modifications, often abbreviated as PTMs, represent a fascinating layer of complexity in the world of proteins. After a protein is synthesized by ribosomes based on the genetic code from mRNA, it doesn’t always stay in its initial form. Instead, it undergoes various chemical changes that fine-tune its structure, function, location, and interactions within the cell. These modifications are crucial for everything from enzyme activation to cell signaling and even responding to environmental stresses.
In this comprehensive article, we’ll dive deep into what PTMs are, why they matter, the different types with real-world examples, how they’re detected, and their roles in diseases. We’ll also explore recent advances in research, drawing from established scientific understanding to provide a clear, accessible overview. Whether you’re a student, researcher, or just curious about biology, this guide aims to break down these concepts in simple, natural terms.
Table of Contents
Imagine a protein as a basic car coming off the assembly line. It has the engine and wheels, but to make it road-ready, you might add paint, tires, or even a turbocharger. PTMs are like those additions or tweaks, happening after the protein is built, and they can dramatically alter how the protein performs. Some occur right away, while others are triggered by specific signals in the cell. This process expands the diversity of proteins far beyond what our genes alone can code for, allowing a single protein to take on multiple roles depending on the context.
What Are Post-Translational Modifications?
Post-translational modification refers to the covalent attachment or alteration of chemical groups to a protein after its synthesis. This happens following the translation of mRNA into a polypeptide chain by ribosomes. These changes can be enzymatic, meaning they’re catalyzed by specific enzymes, or spontaneous, occurring without enzymatic help under certain conditions.
Proteins start as linear chains of amino acids, but PTMs help them fold properly, interact with other molecules, or even get degraded when no longer needed. For instance, many PTMs involve adding groups like phosphates or sugars to specific amino acid side chains, which can switch a protein “on” or “off” like a light switch. This dynamic regulation is essential for maintaining cellular balance, known as homeostasis.
One key aspect is that PTMs are often reversible, allowing cells to respond quickly to changes. However, irreversible ones, like cleavage of peptide bonds, commit the protein to a permanent form. Research shows that over 300 different types of PTMs exist, affecting nearly every protein in some way, highlighting their universal importance in biology.
The Importance of PTMs in Cell Signaling and Beyond
PTMs play a starring role in cell signaling, where cells communicate and coordinate activities. For example, when a hormone binds to a receptor on the cell surface, it often triggers a cascade of PTMs inside the cell, like phosphorylation, to relay the message to the nucleus or other organelles. This is how prohormones get converted into active hormones, ready to perform their duties.
Beyond signaling, PTMs influence protein stability, localization, and interactions. Glycosylation, for one, adds carbohydrate molecules that help proteins fold correctly and protect them from degradation, while also aiding in cell recognition processes, such as in immune responses. Lipidation attaches fatty acids, directing proteins to membranes where they can function effectively.
In stressful conditions, like oxidative stress, PTMs such as carbonylation mark proteins for breakdown, preventing harmful aggregates from building up. This cleanup process is vital to avoid cellular damage. Moreover, PTMs interact with metal ions, regulating functions in signal transduction and gene expression. When these interactions go awry, they can contribute to diseases like cancer or neurodegenerative disorders, where dysregulated PTMs lead to uncontrolled cell growth or protein misfolding.
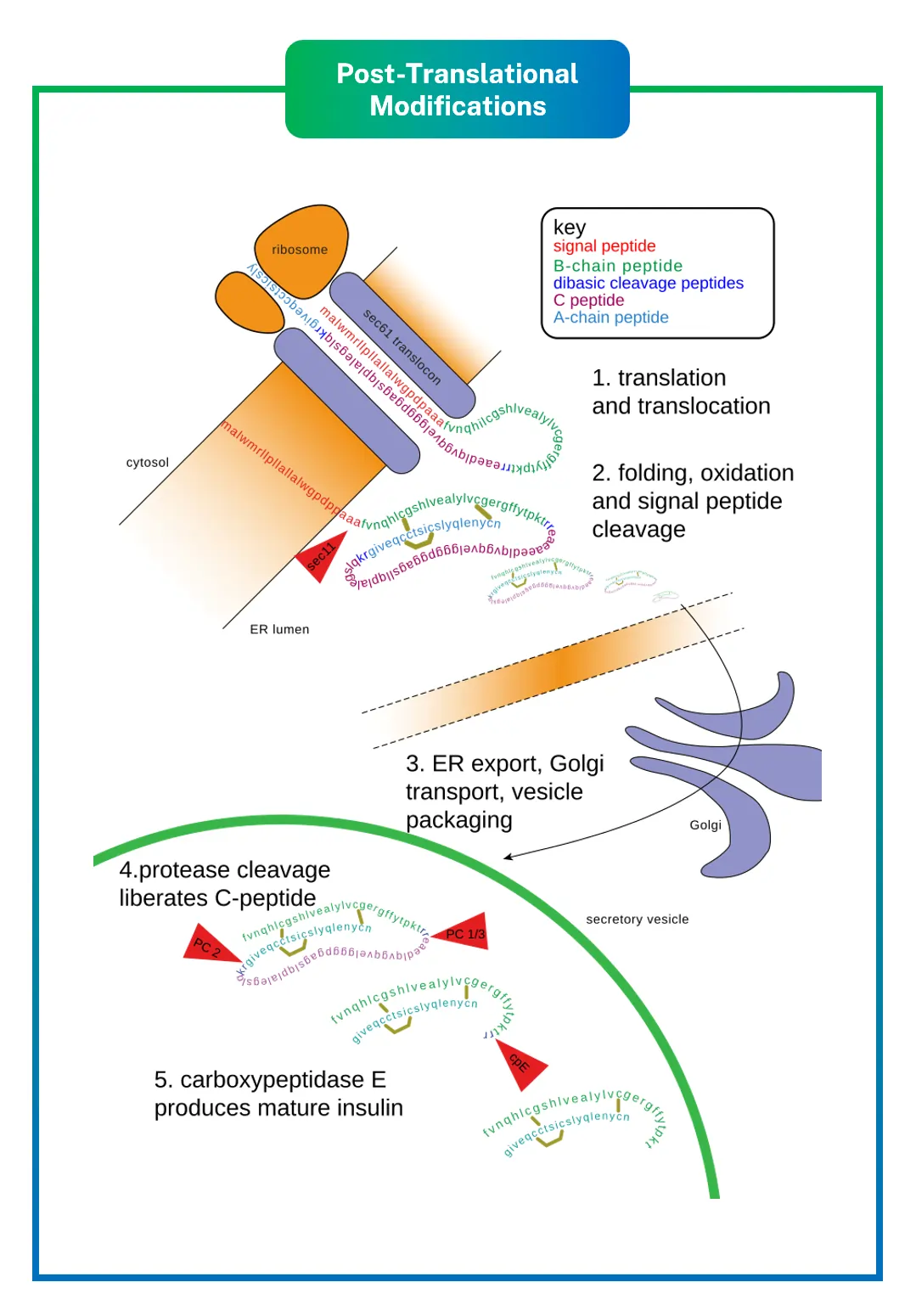
Consider the peptide hormone insulin: after synthesis, it’s modified by forming disulfide bonds and cleaving parts of the chain, resulting in two connected polypeptides that can regulate blood sugar levels. Without these PTMs, insulin wouldn’t work, leading to conditions like diabetes.
Common Sites for Post-Translational Modifications
PTMs don’t happen randomly; they target specific sites on proteins where amino acids have reactive functional groups that act as nucleophiles in chemical reactions. The most common targets include:
- The hydroxyl groups (-OH) on serine, threonine, and tyrosine, which are ideal for additions like phosphorylation or glycosylation.
- The amine groups (-NH2) on lysine, arginine, and histidine, often involved in acetylation, methylation, or ubiquitination.
- The thiol group (-SH) on cysteine, perfect for disulfide bond formation or S-nitrosylation.
- The carboxylate groups (-COOH) on aspartate and glutamate, which can undergo gamma-carboxylation.
- The N- and C-termini of the protein chain, where modifications like amidation or acetylation occur.
Less common sites include the amide group on asparagine for glycan attachment, oxidized methionines, or even methylene groups in side chains. These sites’ reactivity allows precise control over protein function.
For example, in enzymes, modifying a serine residue can create a catalytic site, enhancing activity. In histones, PTMs on lysine tails regulate DNA accessibility, influencing gene expression in epigenetics.
Detection Techniques for PTMs
Identifying PTMs experimentally is crucial for understanding their roles. Common methods include:
- Mass spectrometry: This powerful technique measures the mass-to-charge ratio of protein fragments, detecting added groups by shifts in mass. It’s widely used for mapping multiple PTMs on a single protein.
- Western blotting: Involves separating proteins by gel electrophoresis, transferring to a membrane, and probing with antibodies specific to modified residues, like phospho-specific antibodies.
- Eastern blotting: Similar to Western but focuses on detecting carbohydrate modifications using lectins or other probes.
These techniques have advanced with high-throughput proteomics, allowing researchers to profile PTMs across entire proteomes in health and disease states. For instance, in cancer research, mass spec reveals hyper-phosphorylated proteins driving tumor growth.
Types of PTMs: Addition of Functional Groups
PTMs involving the addition of functional groups are diverse and can be categorized by whether they’re enzymatic (in vivo) or non-enzymatic. These additions expand the protein’s chemical repertoire, enabling new interactions.
Enzymatic Additions In Vivo
Enzymes precisely add groups to regulate protein function. Here’s a breakdown:
Hydrophobic Groups for Membrane Localization
These PTMs anchor proteins to cell membranes by adding lipid moieties:
- Myristoylation: Attaches a C14 saturated fatty acid (myristate) to the N-terminus, often on glycine residues. Example: In Src kinases, this helps signal transduction at the membrane.
- Palmitoylation: Adds a C16 saturated acid (palmitate) via thioester bonds to cysteines. It’s reversible and regulates protein trafficking, as seen in Ras proteins involved in cell growth.
- Isoprenylation (or prenylation): Adds isoprenoid groups like farnesyl (C15) or geranylgeranyl (C20). Farnesylation targets Ras, while geranylgeranylation affects Rho proteins in cytoskeletal organization.
- Glypiation: Forms a glycosylphosphatidylinositol (GPI) anchor at the C-terminus, linking proteins to the outer membrane leaflet. Common in cell surface proteins like alkaline phosphatase.
Cofactors for Enhanced Enzymatic Activity
These attach molecules that boost enzyme performance:
- Lipoylation: Adds a lipoate group (C8) to lysine, essential for pyruvate dehydrogenase in energy metabolism.
- Flavin attachments: Covalent linking of FMN or FAD, as in succinate dehydrogenase for electron transport.
- Heme C attachment: Via thioether bonds to cysteines in cytochromes, crucial for respiration.
- Phosphopantetheinylation: Adds a 4′-phosphopantetheine from CoA, key in fatty acid synthesis pathways.
- Retinylidene Schiff base: Forms in visual pigments like rhodopsin for light detection.
Modifications of Translation Factors
Specialized for proteins in protein synthesis:
- Diphthamide formation on histidine in eEF2, aiding elongation.
- Ethanolamine phosphoglycerol on glutamate in eEF1α.
- Hypusine on lysine in eIF5A, vital for translation in eukaryotes and archaea.
- Beta-lysine addition to elongation factor P in bacteria.
Smaller Chemical Groups
These include:
- Acylation: Forms esters (O-), amides (N-), or thioesters (S-). Acetylation on lysine neutralizes charge, affecting histone-DNA interactions. Deacetylation reverses it.
- Alkylation: Adds alkyl groups; methylation on lysine or arginine regulates epigenetics and signaling.
- Amidation: At C-terminus from glycine oxidation, stabilizing hormones like oxytocin.
- Amide bond formation: Links amino acids or peptides.
- Amino acid addition: Like arginylation, polyglutamylation (on tubulin for microtubule stability), or polyglycylation.
- Butyrylation: Adds butyryl to lysine, similar to acetylation.
- Gamma-carboxylation: Vitamin K-dependent, on glutamate for blood clotting factors.
- Glycosylation: Adds sugars to various residues. O-GlcNAc on serine/threonine competes with phosphorylation in signaling. Polysialylation on NCAM aids neural development.
- Hydroxylation: Oxygen addition to proline/lysine in collagen for structure.
- Iodination: On tyrosine in thyroglobulin for thyroid hormones.
- Nucleotide addition: ADP-ribosylation modifies enzymes like in toxin actions.
- Phosphorylation: Adds phosphate, often to serine/threonine/tyrosine.
- The reaction is: $$ \text{Protein-OH} + \text{ATP} \to \text{Protein-OPO}_3^{2-} + \text{ADP} $$. Highly regulatory, as in MAPK pathways for cell proliferation.
- Adenylylation/Uridylylation: Adds AMP/UMP to tyrosine or others.
- Propionylation: Similar to acetylation on lysine.
- Pyroglutamate formation: Cyclizes N-terminal glutamine.
- S-glutathionylation: Adds glutathione to cysteine for redox protection.
- S-nitrosylation: Adds NO to cysteine, in signaling like vasodilation.
- S-sulfenylation/S-sulfinylation/S-sulfonylation: Oxygen additions to cysteine thiol, from reversible to irreversible, in oxidative stress responses.
- Sulfation: Adds sulfate to tyrosine, in secretory proteins.
Recent studies highlight new PTMs like crotonylation and succinylation on lysine, influencing metabolism and gene regulation in cardiovascular diseases.
Non-Enzymatic Modifications In Vivo
These occur spontaneously:
- Carbamylation: Adds isocyanic acid to N-terminus or lysine.
- Carbonylation: Adds CO, marking for degradation in oxidative stress.
- Glycation: Non-enzymatic sugar attachment, linked to diabetes complications.
- Glutarylation/Malonylation/Methylmalonylation/Succinylation: Acyl additions to lysine, affecting mitochondrial functions.
- Spontaneous isopeptide bonds: In bacterial surface proteins.
Non-enzymatic PTMs like glycoxidation and lipoxidation arise from reactive species, contributing to aging and chronic diseases.
Non-Enzymatic Additions In Vitro
Used in labs or therapeutics:
- Biotinylation: Labels proteins for detection.
- Carbamylation: From urea exposure.
- Oxidation: Affects Met/Trp/His/Cys, forming disulfides.
- Pegylation: Attaches PEG to extend drug half-life, as in interferon therapies.
Conjugation with Other Proteins or Peptides
These PTMs link proteins together:
- Ubiquitination: Attaches ubiquitin, targeting for degradation via proteasome. Polyubiquitination marks for breakdown, while monoubiquitination affects localization.
- SUMOylation: Adds SUMO, regulating nuclear transport and stress responses.
- Neddylation: Adds Nedd8, modulating cullin-RING ligases.
- ISGylation: Adds ISG15, in antiviral responses.
- Pupylation: Prokaryotic ubiquitin-like, in bacteria.
These are key in protein quality control and disease, like in Parkinson’s where faulty ubiquitination leads to aggregates.
Chemical Modifications of Amino Acids
Alter specific residues:
- Citrullination: Converts arginine to citrulline, in inflammation and autoimmunity like rheumatoid arthritis.
- Deamidation: Glutamine to glutamic acid, affecting protein charge.
- Eliminylation: Forms alkenes from phospho-serine/threonine.
Structural Changes
- Disulfide bridges: Cysteine linkages for stability, as in antibodies.
- Lysine-cysteine bridges: NOS/SONOS types in some proteins.
- Proteolytic cleavage: Processes propeptides, like in insulin.
- Isoaspartate formation: From asparagine cyclization, linked to aging.
- Racemization: In peptides like dermorphin.
- Protein splicing: Removes inteins.
PTMs in Health and Disease
PTMs are implicated in numerous diseases. Aberrant phosphorylation drives cancer by activating oncogenes like EGFR. In neurodegenerative disorders like Alzheimer’s, hyper-phosphorylated tau forms tangles, while citrullination and glycosylation alterations affect amyloid processing. Diabetes involves glycation leading to advanced glycation end-products (AGEs) damaging vessels.
In cardiovascular issues, new PTMs like S-palmitoylation regulate ion channels, and dysregulated succinylation contributes to heart failure. Viral infections exploit host PTMs, like ISGylation for immunity.
Databases track disease-associated PTMs, aiding biomarker discovery.
Recent Advances in PTM Research
Advances in mass spectrometry enable proteome-wide PTM mapping, revealing roles in cell death pathways like apoptosis via phosphorylation cascades. Machine learning predicts PTM sites, speeding drug development.
In serum response factor (SRF), PTMs like nitration regulate gene expression in development and disease. Emerging PTMs like lactylation link metabolism to epigenetics.
Comprehensive Table of Common PTMs
To summarize, here’s a detailed table outlining major PTMs, their target amino acids, functions, and examples:
| PTM Type | Target Amino Acids | Key Functions | Examples in Biology | Associated Enzymes/Processes | Disease Links |
|---|---|---|---|---|---|
| Phosphorylation | Serine, Threonine, Tyrosine | Regulates activity, signaling | MAPK pathway activation | Kinases (e.g., PKA), Phosphatases | Cancer (hyper-phosphorylation), Alzheimer’s (tau) |
| Glycosylation | Asparagine, Serine, Threonine | Protein folding, stability, recognition | Antibody glycosylation | Glycosyltransferases | Congenital disorders of glycosylation, Cancer |
| Acetylation | Lysine | Gene regulation, charge neutralization | Histone acetylation for transcription | Acetyltransferases (HATs), Deacetylases (HDACs) | Cancer, Neurodegenerative diseases |
| Methylation | Lysine, Arginine | Epigenetic control, signaling | Histone methylation | Methyltransferases | Cancer (epigenetic silencing) |
| Ubiquitination | Lysine | Degradation, localization | p53 ubiquitination | E1/E2/E3 ligases | Parkinson’s, Cancer |
| SUMOylation | Lysine | Nuclear transport, stress response | PML protein in nuclear bodies | SUMO ligases | Cancer, Viral infections |
| Palmitoylation | Cysteine | Membrane anchoring | Ras protein localization | Palmitoyltransferases | Cardiovascular diseases |
| Myristoylation | Glycine (N-terminal) | Membrane association | Src kinase | N-myristoyltransferase | Viral assembly issues |
| ADP-ribosylation | Various | Signaling, DNA repair | PARP in DNA damage response | ADP-ribosyltransferases | Cancer therapies (PARP inhibitors) |
| Citrullination | Arginine | Inflammation regulation | Myelin basic protein in MS | Peptidylarginine deiminases (PADs) | Rheumatoid arthritis, Multiple sclerosis |
| Succinylation | Lysine | Metabolic regulation | Mitochondrial enzymes | Succinyltransferases | Metabolic disorders, Heart disease |
| Crotonylation | Lysine | Gene expression | Histone crotonylation | Crotonyltransferases | Kidney diseases, Cancer |
| S-nitrosylation | Cysteine | Redox signaling | Hemoglobin in vasodilation | Nitric oxide synthases | Neurodegenerative, Cardiovascular |
| Carbonylation | Various | Oxidative stress marker | Protein aggregates in aging | Spontaneous (ROS-induced) | Aging, Diabetes |
This table captures the breadth of PTMs, showing how they interconnect functions across cellular processes.
Expanded Table of PTMs in Specific Diseases
For a deeper look at disease implications, consider this table:
| Disease Category | Key PTMs Involved | Affected Proteins/Processes | Mechanisms | Potential Biomarkers/Therapies |
|---|---|---|---|---|
| Cancer | Phosphorylation, Ubiquitination, Acetylation | Oncogenes like EGFR, Tumor suppressors like p53 | Hyper-activation of growth signals, Evaded degradation | Phospho-specific antibodies, HDAC inhibitors |
| Alzheimer’s Disease | Phosphorylation, Glycosylation, Citrullination | Tau, Amyloid-beta | Tangle formation, Plaque accumulation | Mass spec profiling, Anti-tau drugs |
| Cardiovascular | Succinylation, Palmitoylation, Crotonylation | Ion channels, Metabolic enzymes | Dysregulated energy, Membrane dysfunction | Enzyme inhibitors, Lifestyle interventions |
| Diabetes | Glycation, Carbonylation | Hemoglobin, Vascular proteins | AGE formation, Oxidative damage | HbA1c monitoring, Antioxidants |
| Autoimmune (e.g., RA) | Citrullination, Methylation | Joint proteins, Histones | Autoantibody generation, Inflammation | PAD inhibitors, DMARDs |
| Viral Infections | ISGylation, SUMOylation | Host defense proteins | Antiviral signaling enhancement | Interferon therapies |
These tables illustrate the clinical relevance, guiding ongoing research.
In conclusion, post-translational modifications are the unsung heroes of protein biology, adding layers of control that make life possible. From simple additions to complex conjugations, they ensure proteins adapt to ever-changing cellular needs. As research progresses with tools like advanced mass spectrometry and AI predictions, we’re uncovering more about their roles in health and disease, paving the way for targeted therapies. Understanding PTMs not only deepens our appreciation of molecular biology but also holds promise for treating complex conditions.
Frequently Asked Questions
FAQ 1: What Are Post-Translational Modifications and How Do They Influence Protein Diversity?
Post-translational modifications are chemical changes that happen to proteins after they’ve been synthesized in the cell, essentially adding a layer of customization to make them more versatile. Think of a protein as a basic tool fresh from the factory; PTMs are like attaching handles, sharpening edges, or even combining it with other parts to suit specific jobs. These modifications can involve adding groups like phosphates, sugars, or lipids, or even cleaving parts of the protein chain. They occur either through enzymes that catalyze the process or spontaneously under certain cellular conditions, and they’re vital because they expand the functional range of proteins beyond what the genetic code alone provides.
In everyday cellular life, PTMs help proteins interact with each other, move to the right locations, and respond to signals. For instance, in cell signaling, a hormone might trigger a series of PTMs that activate enzymes, leading to changes in metabolism or gene expression. Without these modifications, proteins might not fold properly, could degrade too quickly, or fail to form necessary complexes. Research has shown that PTMs are involved in nearly every biological process, from immune responses to development, and their dysregulation can lead to serious issues like diseases. Moreover, with over 300 known types, PTMs create immense diversity; a single protein can have multiple forms, each with unique roles depending on the modification.
This diversity is particularly crucial in complex organisms where the number of genes is limited, but the proteome—the full set of proteins—needs to be expansive. PTMs allow for quick adaptations to environmental changes, such as stress or nutrient availability. In health contexts, they maintain balance, but in diseases, altered PTMs can disrupt this harmony, leading to aggregates in neurodegenerative conditions or uncontrolled growth in cancer. Ongoing studies continue to uncover how these modifications interplay with metal ions or oxidative stress, further emphasizing their role in cellular homeostasis and potential as therapeutic targets.
FAQ 2: How Do Post-Translational Modifications Affect Protein Function in Cells?
Post-translational modifications profoundly impact how proteins work within cells by altering their structure, activity, and interactions. At a basic level, adding a small group like a phosphate can change a protein’s charge, making it more or less likely to bind to partners or enter certain cellular compartments. This is akin to flipping a switch: the modification turns the protein on or off for specific tasks. For example, in enzyme regulation, phosphorylation often activates or inhibits catalytic sites, controlling pathways like glucose metabolism or cell division.
These changes also influence protein stability and lifespan. Some PTMs mark proteins for degradation, ensuring old or damaged ones are removed, while others stabilize them against breakdown. In signaling cascades, a chain of PTMs can amplify a message from the cell surface to the nucleus, coordinating responses to stimuli. Glycosylation, another common modification, adds sugars that help proteins fold correctly and interact with the immune system, preventing misrecognition as foreign invaders.
Beyond individual functions, PTMs enable proteins to multitask. A protein might be inactive in one form but, after modification, relocate to the membrane or join a complex. This flexibility is essential for dynamic processes like apoptosis or immune activation. However, when PTMs go wrong—due to mutations in modifying enzymes or environmental factors—they can lead to dysfunction, as seen in various pathologies where hyper-modified proteins accumulate or fail to signal properly.
FAQ 3: What Role Do Post-Translational Modifications Play in Cancer Development?
Post-translational modifications are key players in cancer, often driving the transformation of normal cells into malignant ones by altering critical proteins involved in growth and survival. In many cancers, aberrant PTMs like hyper-phosphorylation activate oncogenes, proteins that promote uncontrolled cell division. For instance, in breast cancer, acetylation of certain transcription factors enhances their activity, leading to overexpression of genes that fuel tumor growth. These modifications don’t just affect one pathway; they create a web of changes that allow cancer cells to evade death, invade tissues, and resist treatments.
- Phosphorylation in signaling pathways: This PTM often overactivates kinases like EGFR, promoting proliferation in lung and colorectal cancers. The reaction for phosphorylation is typically $$ \text{Protein-OH} + \text{ATP} \to \text{Protein-OPO}_3^{2-} + \text{ADP} $$, catalyzed by kinases, and its dysregulation leads to persistent signaling.
- Ubiquitination and degradation: Faulty ubiquitination fails to degrade tumor suppressors like p53, allowing damaged cells to survive and mutate further, common in various carcinomas.
- Acetylation and epigenetics: Hyper-acetylation of histones loosens DNA, increasing access to oncogenes, while deacetylation silences suppressors, seen in leukemia.
- Glycosylation alterations: Abnormal sugar additions to proteins aid metastasis by changing cell adhesion, as in pancreatic cancer where it promotes invasion.
These PTM changes provide cancer cells with metabolic advantages, such as enhanced glycolysis, and immune evasion. Targeting PTM enzymes, like HDAC inhibitors for acetylation, has emerged as a therapeutic strategy, showing promise in clinical trials by restoring normal modification patterns and halting tumor progression.
FAQ 4: How Are Post-Translational Modifications Involved in Neurodegenerative Diseases?
In neurodegenerative diseases, post-translational modifications often go awry, leading to protein misfolding, aggregation, and neuronal damage. Take Alzheimer’s disease, for example: hyper-phosphorylation of tau protein causes it to detach from microtubules and form tangles, disrupting cellular transport and leading to cell death. This modification, meant to regulate tau’s function, becomes excessive due to imbalanced kinases and phosphatases, exacerbating plaque formation alongside amyloid-beta. Similarly, in Parkinson’s, alpha-synuclein undergoes ubiquitination and phosphorylation, promoting Lewy body aggregates that impair dopamine-producing neurons.
These PTMs aren’t just markers; they actively contribute to disease progression. Oxidative stress-induced carbonylation tags proteins for degradation, but in overwhelmed systems, it leads to harmful buildup. Citrullination, converting arginine to citrulline, alters myelin proteins in multiple sclerosis, triggering autoimmune attacks. In Huntington’s, polyglutamylation on huntingtin extends glutamine tracts, enhancing toxicity and aggregation.
Therapeutically, understanding these modifications opens doors to interventions. Inhibitors targeting overactive kinases could reduce tau phosphorylation, while enhancing deubiquitinating enzymes might clear aggregates. Recent research highlights how PTMs interact with aging processes, where reduced efficiency in modification reversal accelerates neurodegeneration, emphasizing the need for early detection through PTM profiling.
FAQ 5: What Are Some Recent Advances in Research on Post-Translational Modifications?
| Advance Category | Description | Key Examples | Impact on Research and Medicine |
|---|---|---|---|
| Mass Spectrometry Improvements | Enhanced techniques like data-independent acquisition allow deeper PTM mapping in complex samples. | Studies on Alzheimer’s PTMs using DDA and DIA for phosphorylation and glycosylation analysis. | Enables proteome-wide insights, aiding biomarker discovery for diseases. |
| AI and Machine Learning Integration | Tools predict PTM sites and structural impacts, speeding up analysis. | Leveraging AI to explore PTM contexts in therapeutics. | Accelerates drug design by simulating modifications. |
| Database Updates | Comprehensive resources like dbPTM 2025 integrate PTMs with cancer data. | Over 300 PTM types cataloged for functional studies. | Facilitates global research collaboration and hypothesis generation. |
| Novel PTM Discoveries | Identification of hybrid modifications like poly-ubiquitin chains. | New hybrid PTMs in protein regulation. | Reveals untapped regulatory mechanisms in signaling. |
| Therapeutic Engineering | Modifying PTMs for stability in biologics. | Engineering myristoylation or glycosylation in peptides. | Improves drug efficacy for chronic conditions. |
| Metabolite-Induced PTMs | Linking metabolism to PTMs in transcription factors. | Metabolite-driven modifications in disease models. | Offers perspectives on metabolic disorders and cancer. |
| Glycosylation in Development | Dysregulation linked to organ-specific issues. | New types in cardiovascular contexts. | Guides targeted therapies for developmental diseases. |
FAQ 6: What Are Some Uncommon Post-Translational Modifications and Their Biological Significance?
Uncommon post-translational modifications add intriguing twists to protein function, often overlooked but crucial in niche cellular processes. For example, ADP-ribosylation attaches ADP-ribose units, regulating DNA repair and stress responses; its dysregulation can lead to genomic instability in aging cells. Another rare one is succinylation, adding a succinyl group to lysine, influencing mitochondrial metabolism and linked to energy disorders.
- Citrullination: Converts arginine to citrulline, affecting protein charge and common in inflammation; it’s significant in autoimmune diseases like rheumatoid arthritis, where it triggers antibody responses.
- Glutarylation: A lysine modification impacting chromatin and gene expression, with roles in neurological functions and potential ties to brain disorders.
- Eliminylation: Forms alkenes from phosphorylated residues, rare but involved in signaling under stress conditions.
- Isoaspartate formation: Arises from asparagine deamidation, associated with protein aging and cataracts, highlighting longevity impacts.
These lesser-known PTMs expand our understanding of protein dynamics, offering new avenues for diagnostics in subtle pathologies.
FAQ 7: What Advancements Have Been Made in Techniques for Detecting Post-Translational Modifications?
Detecting post-translational modifications has seen remarkable progress, transforming from labor-intensive methods to high-throughput, precise technologies. Mass spectrometry remains the gold standard, with advancements like data-independent acquisition allowing simultaneous analysis of thousands of PTMs without prior selection, capturing fleeting modifications in real-time cellular contexts. This has been pivotal in blood cancer research, where PTM profiles reveal therapeutic targets.
Enrichment strategies have evolved, using antibodies or chemical tags to isolate modified peptides, enhancing sensitivity for low-abundance PTMs. Machine learning models now predict modification sites, integrating with MS data for faster interpretation. Gel-based staining for direct visualization of phosphorylations adds simplicity for initial screens.
These tools not only map PTMs but quantify their dynamics, linking them to disease states and paving the way for personalized medicine through PTM-based biomarkers.
FAQ 8: Can You Provide Examples of Post-Translational Modifications in Viral Infections?
| PTM Type | Virus Example | Affected Protein/Process | Mechanism and Impact |
|---|---|---|---|
| Ubiquitination | Influenza | Host immune proteins | Virus hijacks ubiquitination to degrade antiviral factors, enhancing replication. |
| Phosphorylation | HIV | Tat protein | Phosphorylation at serine-16 regulates viral transcription, boosting genome production. |
| Glycosylation | Hepatitis B | Viral envelope proteins | Aids immune evasion and assembly, critical for chronic infection. |
| SUMOylation | Dengue | Envelope protein | Promotes egress in mosquito cells, affecting transmission. |
| ADP-ribosylation | Zika | NS1 and NS3 | Host PARP12 adds groups, leading to degradation and restricting spread. |
| Acetylation | Lassa | Host receptors | Alters avidity, influencing entry pathways. |
| ISGylation | Various RNA viruses | Viral proteins | Enhances antiviral responses by modifying host defenses. |
| Palmitoylation | SARS-CoV-2 | Spike protein | Facilitates membrane fusion and entry. |
FAQ 9: How Do Post-Translational Modifications Contribute to Metabolic Disorders?
Post-translational modifications are intricately linked to metabolic disorders, regulating enzymes and pathways that control glucose and lipid balance. In diabetes, for instance, glycosylation of hemoglobin forms HbA1c, a marker of chronic high blood sugar, while phosphorylation of insulin receptors can impair signaling, leading to resistance.
- Acetylation in obesity: Hyper-acetylation of metabolic enzymes disrupts fat breakdown, promoting accumulation and related complications like fatty liver.
- Ubiquitination in hyperlipidemia: Dysregulated ubiquitination affects cholesterol regulators, causing buildup and cardiovascular risks.
- Phosphorylation in insulin resistance: Over-phosphorylation of key kinases like AMPK alters energy sensing, exacerbating type 2 diabetes.
- Succinylation in mitochondrial dysfunction: Impacts electron transport, linking to metabolic syndromes and organ failures.
Targeting these PTMs with inhibitors offers hope for treatments, restoring metabolic harmony.
FAQ 10: What Are the Therapeutic Implications of Targeting Post-Translational Modifications?
Targeting post-translational modifications holds immense promise for treating diseases by correcting aberrant protein changes. In cancer, inhibitors of kinases that drive phosphorylation have revolutionized therapy, like imatinib for leukemia, which blocks overactive signaling and induces remission. Similarly, HDAC inhibitors reverse faulty acetylation in epigenetics, reactivating suppressed genes to halt tumor growth.
For neurodegenerative conditions, modulating ubiquitination enzymes could clear aggregates, as in Parkinson’s where enhancing deubiquitinases reduces alpha-synuclein toxicity. In metabolic disorders, drugs influencing glycosylation might mitigate diabetes complications by preventing advanced glycation end-products.
These approaches extend to engineering biologics with stable PTMs for longer efficacy, or using small molecules to induce beneficial modifications. Challenges remain in specificity to avoid off-target effects, but with advancing proteomics, personalized PTM-targeted therapies are on the horizon, potentially transforming chronic disease management.
FAQ 11: What Is the Role of Post-Translational Modifications in the Immune Response?
Post-translational modifications play a pivotal role in shaping the immune response, acting as rapid switches that fine-tune protein functions during infection or inflammation. In innate immunity, PTMs like phosphorylation activate key transcription factors such as IRF-3, enabling quick gene expression for antiviral cytokines. This enzymatic process allows cells to respond swiftly to pathogens without needing new protein synthesis, ensuring a timely defense. For instance, in response to viral infections, modifications like ubiquitination mark viral proteins for degradation, while SUMOylation regulates inflammatory pathways to prevent overactivation that could lead to tissue damage.
Beyond innate responses, PTMs influence adaptive immunity by modulating T-cell and B-cell activities. Acetylation on histones opens chromatin for antibody gene rearrangement, while glycosylation on immune checkpoints like PD-1 affects their stability and interaction with ligands, impacting T-cell exhaustion in chronic infections or cancer. These modifications also create neoepitopes, potentially triggering autoimmunity if dysregulated, as seen in conditions where citrullination breaks immune tolerance. Metabolite-driven PTMs, such as succinylation, further integrate metabolism with immunity, altering macrophage polarization during inflammation.
Overall, PTMs ensure a balanced immune response, but their dysregulation can lead to immunodeficiencies or hyperinflammation. Emerging research highlights unconventional PTMs, like those induced by oxidative stress, which enhance pattern recognition receptors’ sensitivity to environmental cues, offering new therapeutic avenues for modulating immunity in diseases like sepsis or autoimmunity.
FAQ 12: How Have Post-Translational Modifications Evolved Over Time?
| Evolutionary Aspect | Description | Key Examples | Implications for Biology |
|---|---|---|---|
| Origin in Prokaryotes | PTMs likely began in simple organisms for basic regulation, with phosphorylation emerging early for signaling. | Bacterial kinases modifying response regulators in two-component systems. | Laid foundation for complex cellular responses. |
| Expansion in Eukaryotes | Increased PTM diversity with compartmentalization, enabling finer control over gene expression and interactions. | Histone acetylation and methylation for epigenetic regulation. | Allowed multicellularity and adaptation. |
| Cross-Talk Development | Evolution of PTM interactions, where one influences another, generating phenotypic diversity. | Ubiquitination affecting phosphorylation sites in signaling pathways. | Enhanced rapid evolutionary changes without genetic mutations. |
| Role in Divergence | PTMs drive species-specific traits by altering protein functions post-translationally. | Variations in glycosylation patterns between primates and humans. | Contributes to speciation and disease susceptibility. |
| Modern Complexity | Over 300 PTMs now known, with enzymes evolving specificity. | SUMOylation in stress responses across kingdoms. | Supports advanced processes like neural development. |
| Future Insights | Ongoing studies reveal PTM roles in fast adaptation. | PTMs in viral evolution and host defense. | Potential for synthetic biology applications. |
FAQ 13: What Functions Do Post-Translational Modifications Serve in Plant Biology?
Post-translational modifications are essential in plant biology, helping plants adapt to their environment without mobility. They regulate processes like photosynthesis, hormone signaling, and defense against herbivores or pathogens. For example, phosphorylation activates enzymes in light-harvesting complexes, optimizing energy capture under varying light conditions.
- Stress Response: PTMs like ubiquitination target damaged proteins for degradation during drought or salt stress, maintaining cellular homeostasis.
- Growth and Development: Glycosylation stabilizes cell wall proteins, aiding root elongation and leaf expansion.
- Pathogen Defense: Acetylation modifies resistance proteins, enhancing recognition of invaders and triggering hypersensitive responses.
- Metabolic Regulation: Succinylation in mitochondria fine-tunes enzyme activity for nutrient uptake.
These modifications allow dynamic responses, with databases like qPTMplants cataloging them for research into crop improvement.
FAQ 14: What Are Some Bioinformatics Tools for Predicting Post-Translational Modifications?
| Tool Name | Description | Key Features | Applications |
|---|---|---|---|
| MusiteDeep | Deep-learning webserver for PTM site prediction and visualization. | Handles phosphorylation, acetylation; provides confidence scores. | Proteome-wide analysis in disease studies. |
| PTM-ssMP | Predicts multiple PTMs like phosphorylation, ubiquitination. | User-friendly interface; integrates sequence motifs. | Multi-PTM crosstalk exploration. |
| MIND-S | Protein-level prediction using sequence and structure. | Incorporates 3D data for accuracy. | Structural biology and drug design. |
| Sitetack | Improves PTM prediction with known modifications. | Focuses on context-aware deep learning. | Enhancing existing datasets. |
| PTMscape | Open-source for generic PTM prediction and enrichment. | Tests PTM pairs in domains. | Functional association studies. |
| GPS | Group-based system for various PTMs. | High specificity for kinases. | Signaling pathway mapping. |
| PTMcode | Database of PTM functional associations. | Predicts crosstalk within proteins. | Evolutionary and interaction analyses. |
FAQ 15: How Do Post-Translational Modifications Contribute to the Aging Process?
Post-translational modifications increasingly accumulate with age, linking oxidative stress, inflammation, and cellular decline. Non-enzymatic PTMs like glycation form advanced glycation end-products, stiffening tissues and promoting chronic inflammation in vessels and organs. This contributes to age-related diseases by impairing protein function and triggering immune responses.
In skeletal muscle, aging-related PTMs alter myosin, affecting contractility and leading to sarcopenia. Phosphorylation and acetylation shifts disrupt energy metabolism, while carbonylation marks proteins for inefficient degradation, fostering aggregates like those in Alzheimer’s. Epigenetic PTMs on histones also change, silencing repair genes and accelerating senescence.
These modifications serve as biomarkers, with proteomic profiling revealing patterns in long-lived species. Interventions targeting PTM enzymes, like sirtuins for deacetylation, show promise in extending healthspan by restoring youthful protein dynamics.
FAQ 16: How Do Environmental Factors Influence Post-Translational Modifications?
Environmental factors profoundly affect post-translational modifications, altering protein behavior in response to external cues. Pollutants or UV radiation induce oxidative PTMs like carbonylation, marking proteins for degradation but potentially overwhelming cells in chronic exposure.
- Temperature Stress: Heat shocks trigger phosphorylation cascades, activating chaperones for protein refolding.
- Nutrient Availability: Starvation promotes acetylation changes in metabolic enzymes, shifting energy pathways.
- Toxins and Pollutants: Heavy metals cause S-glutathionylation, protecting against oxidative damage but disrupting signaling if persistent.
- Pathogen Exposure: Infections induce ubiquitination for immune activation.
These influences highlight PTMs as adaptive mechanisms, with implications for environmental health studies.
FAQ 17: What Role Do Post-Translational Modifications Play in Stem Cell Differentiation?
| PTM Type | Role in Differentiation | Key Examples | Therapeutic Potential |
|---|---|---|---|
| Phosphorylation | Regulates transcription factors for lineage commitment. | Oct4 phosphorylation promoting neural differentiation. | Targeting kinases for regenerative medicine. |
| Acetylation | Alters chromatin accessibility for gene activation. | Histone acetylation in mesenchymal stem cells. | HDAC inhibitors to enhance pluripotency. |
| Ubiquitination | Controls protein stability in pluripotency maintenance. | Nanog degradation during exit from stemness. | Modulating for cancer stem cell therapies. |
| Glycosylation | Influences cell surface markers and signaling. | O-GlcNAc on Sox2 for embryonic development. | Biomarkers for differentiation stages. |
| SUMOylation | Fine-tunes nuclear transport in fate decisions. | SUMO on Lin28 for metabolic shifts. | Potential in tissue engineering. |
FAQ 18: What Is Crosstalk Between Different Post-Translational Modifications?
Crosstalk between post-translational modifications occurs when one PTM influences another’s addition, removal, or effect, creating complex regulatory networks. For example, phosphorylation can prime a site for ubiquitination, leading to protein degradation, as in cell cycle control where this interplay ensures timely progression.
In chromatin, acetylation and methylation compete on lysine residues, with acetylation promoting open structures for transcription and methylation potentially repressing it. This dynamic forms a “PTM code” that cells read for precise responses. SUMOylation often antagonizes ubiquitination, stabilizing proteins in stress conditions.
Such interactions extend to phase separation, where PTMs drive condensate formation for signaling hubs. Dysregulated crosstalk contributes to diseases, offering targets for therapies that modulate multiple PTMs simultaneously.
FAQ 19: How Do Post-Translational Modifications Contribute to Drug Resistance?
Post-translational modifications are central to drug resistance, modifying proteins to evade therapeutic effects. In cancer, phosphorylation activates efflux pumps, expelling chemotherapy drugs.
- Ubiquitination: Alters degradation of resistance factors, prolonging their activity in bacterial infections.
- Acetylation: Epigenetically silences apoptosis genes, allowing survival under treatment.
- Glycosylation: Modifies surface proteins for immune evasion in resistant tumors.
- Citrullination: Enhances metastasis in resistant colorectal cancer cells.
Targeting PTM enzymes reverses resistance, improving outcomes.
FAQ 20: What Are the Future Research Trends in Post-Translational Modifications?
| Trend | Description | Emerging Technologies | Potential Impacts |
|---|---|---|---|
| AI Integration | Using machine learning for PTM prediction and interpretation. | Prompt-based models like PTMGPT2. | Faster biomarker discovery. |
| Multi-Omics Approaches | Combining PTM data with genomics and metabolomics. | Advanced MS for Alzheimer’s PTMs. | Holistic disease understanding. |
| Spray-Type Modifications | Local-dependent PTMs in spatial contexts. | Imaging and single-cell analysis. | Precision therapeutics. |
| Enrichment Methods | Improved techniques for rare PTM detection. | Affinity-based enrichments. | Broader proteome coverage. |
| Cancer Immunotherapy | Targeting PTMs in tumor microenvironments. | Modifying checkpoints for efficacy. | Enhanced treatments. |
| Sepsis and Inflammation | Ubiquitination patterns in immune responses. | High-throughput screening. | Novel anti-inflammatory drugs. |
Acknowledgments
The development of the article “Post-Translational Modifications: Types, Functions, and Key Examples” was made possible through the wealth of knowledge provided by several authoritative sources. The Examsmeta.com website is deeply grateful for the comprehensive insights and up-to-date research accessible on UniProt (www.uniprot.org/), which offered detailed protein modification data; PubMed (pubmed.ncbi.nlm.nih.gov), which provided access to cutting-edge studies on PTMs in health and disease; Nature (www.nature.com), whose in-depth articles enriched the understanding of PTM roles in cellular processes; and ScienceDirect (www.sciencedirect.com), which supplied critical reviews on PTM detection techniques and their implications. These platforms were instrumental in ensuring the article’s accuracy and depth.
Key contributions include:
- UniProt: Provided extensive databases on protein modifications and their functional annotations.
- PubMed: Offered peer-reviewed studies on PTM roles in diseases like cancer and neurodegeneration.
- Nature: Contributed insights into PTM crosstalk and evolutionary perspectives.
- ScienceDirect: Supplied detailed methodologies for PTM detection and therapeutic applications.

