Autocrine signaling represents a fascinating aspect of how cells communicate within the body. In the intricate world of cellular interactions, cells don’t just send messages to distant neighbors or nearby companions; sometimes, they talk to themselves. This self-directed communication allows cells to regulate their own behavior, respond to internal needs, and adapt to changing environments. Imagine a cell whispering instructions to itself to grow, divide, or even defend against threats. This process is essential for maintaining balance in tissues and organs, and it plays a pivotal role in both health and disease.
As we delve deeper into this topic, we’ll explore its definitions, characteristics, mechanisms, and real-world applications, drawing from established biological principles to provide a thorough understanding.
Table of Contents
In biological systems, communication between cells ensures coordinated functions, from growth and repair to immune responses. While many signaling pathways involve multiple cells, autocrine signaling stands out because it involves a single cell acting as both the sender and receiver. This form of signaling is not limited to humans; it’s observed across various organisms, including animals and even some plants, where cells release chemical messengers that bind back to their own surface receptors. This self-stimulation helps cells fine-tune their activities without relying on external cues, making it a cornerstone of cellular autonomy.
The concept of autocrine signaling was first recognized in the context of growth factors and hormones, where scientists noticed that certain cells could stimulate their own proliferation. Over time, research has expanded our knowledge, revealing its involvement in diverse processes like tissue development and pathological conditions. By understanding this signaling, we gain insights into how the body maintains homeostasis and how disruptions can lead to disorders.
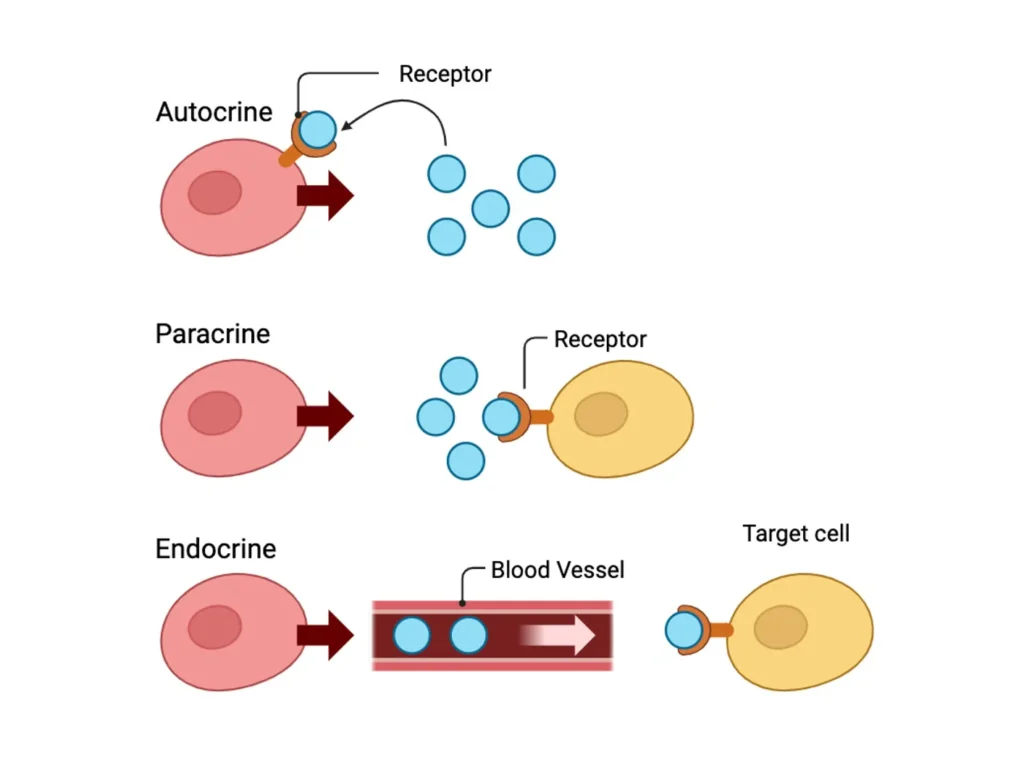
What is Autocrine Signaling?
Autocrine signaling is a type of cell communication where a cell produces and releases signaling molecules, known as ligands or autocrine agents, that then bind to receptors on the very same cell. This creates a feedback loop that allows the cell to influence its own functions. Unlike other forms of signaling that span distances or involve adjacent cells, autocrine signals are inherently local and self-targeted.
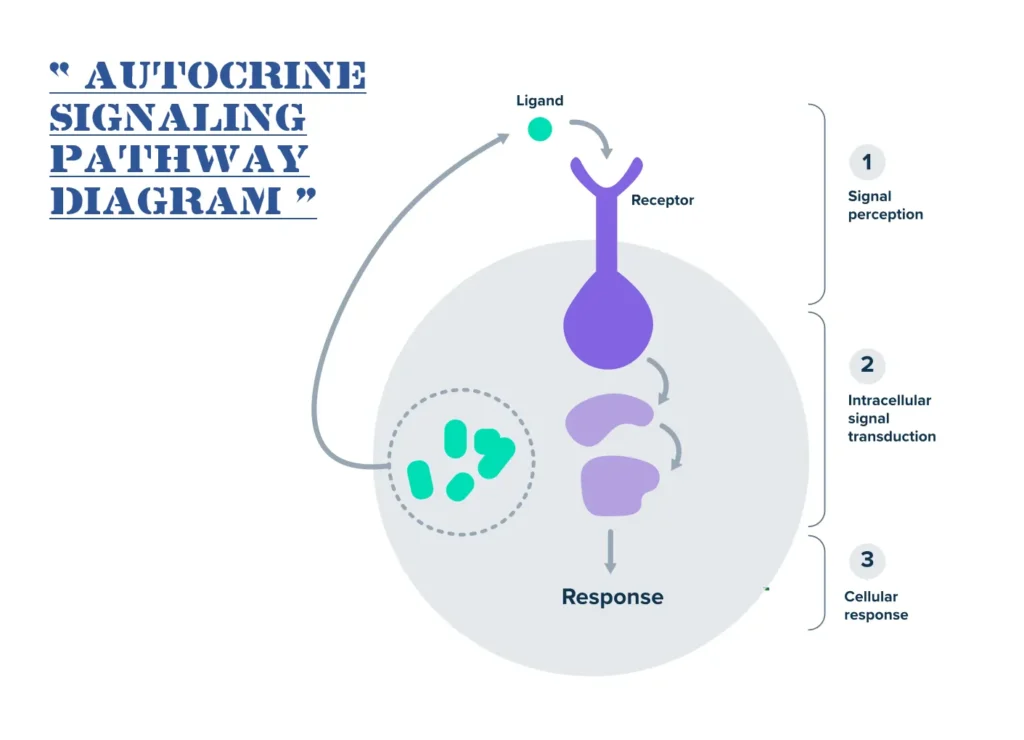
Consider a cell in need of rapid adjustment, such as during stress or growth phases. It secretes specific chemicals—often hormones, cytokines, or growth factors—that interact with its own surface proteins called receptors. Once bound, these interactions trigger a cascade of internal events, leading to changes in gene expression, metabolism, or behavior. This process is aptly termed “self-signaling” because the cell is essentially commanding itself.
In evolutionary terms, autocrine signaling likely developed as a mechanism for single-celled organisms to regulate their internal states before multicellular life evolved. In complex organisms today, it integrates with broader signaling networks, ensuring that individual cells can operate independently when necessary. For instance, in densely packed tissues, this self-reliance prevents over-dependence on neighboring cells, promoting efficiency.
Scientists classify the chemicals involved as autocrine chemicals, which include a variety of molecules like interleukins in immune cells or epidermal growth factors in epithelial tissues. The receptors, dubbed autocrine receptors, are specialized to recognize these self-produced signals, ensuring specificity and preventing cross-talk with other pathways.
Key Characteristics of Autocrine Signaling
Autocrine signaling exhibits several distinctive features that set it apart from other cellular communication methods. These traits highlight its efficiency and role in precise self-regulation.
- The source and target are identical: The cell producing the signal is the same one that responds to it, creating a closed-loop system.
- Short lifespan of signals: Autocrine agents often degrade quickly, ensuring rapid and transient effects to avoid prolonged activation.
- Independence from transport mediums: Unlike hormones in endocrine signaling that travel via bloodstream, autocrine signals diffuse locally without needing vessels.
- Context-dependent activation: Signals are typically released in response to specific internal or environmental triggers, such as nutrient availability or injury.
- Potential for amplification: A single signal can lead to amplified responses through intracellular pathways, allowing small inputs to yield significant outputs.
These characteristics make autocrine signaling ideal for scenarios requiring quick, self-contained adjustments. For example, in a healing wound, cells might use this to proliferate without waiting for signals from distant sources.
Furthermore, the signaling’s brevity prevents chronic overstimulation, which could otherwise lead to uncontrolled growth. In healthy systems, this balance is meticulously maintained through regulatory proteins that modulate receptor sensitivity.
The Importance of Autocrine Signaling in Biological Systems
The significance of autocrine signaling cannot be overstated, as it underpins many fundamental biological processes. In a body composed of trillions of cells, each must function autonomously yet harmoniously. This signaling provides the autonomy, allowing cells to self-regulate without a central controller, much like how individual musicians in an orchestra adjust their play based on self-heard notes.
One key role is in cellular maintenance and repair. Cells constantly face wear and tear, and autocrine signaling enables them to initiate repairs independently. This is crucial in tissues with low turnover rates, where waiting for external signals could delay recovery.
In development, this signaling guides embryonic cells to differentiate and organize into structures. Without it, coordinated growth would falter, leading to developmental abnormalities.
Moreover, autocrine signaling bolsters the immune system by empowering cells to amplify their responses during infections. It also contributes to metabolic regulation, where cells adjust energy production based on self-assessed needs.
Disruptions in this process can have profound implications. Overactive autocrine loops are implicated in diseases like cancer, where cells ignore regulatory checks. Conversely, deficient signaling might impair wound healing or immune function. Thus, understanding its importance aids in developing targeted therapies.
From a broader perspective, autocrine signaling exemplifies nature’s efficiency, allowing complex systems to emerge from simple self-interactions. It’s a testament to how evolution favors mechanisms that enhance survival through self-sufficiency.
Mechanism of Autocrine Signaling
The mechanism of autocrine signaling follows a straightforward yet intricate sequence, ensuring precise control over cellular responses. It begins with the synthesis and release of the signaling molecule inside the cell.
First, in response to an internal stimulus—like DNA damage or nutrient scarcity—the cell transcribes genes to produce the autocrine agent. This molecule is then packaged and secreted into the extracellular space, often via exocytosis.
Once outside, the ligand diffuses briefly before binding to receptors on the same cell’s membrane. These receptors are typically transmembrane proteins, such as G-protein-coupled receptors or tyrosine kinases, designed to recognize specific ligands.
Upon binding, the receptor undergoes a conformational change, activating intracellular signaling pathways. This might involve second messengers like cyclic AMP or phosphorylation cascades that amplify the signal.
The amplified signal reaches the nucleus or other organelles, altering gene expression, protein synthesis, or metabolic activity. For instance, it could upregulate genes for cell division.
Finally, feedback mechanisms terminate the signal: Ligands degrade, receptors desensitize, or inhibitory proteins intervene, preventing overstimulation.
In some cases, cells recycle the components, storing ligands for future use, which is efficient for repetitive tasks.
This mechanism’s elegance lies in its speed and locality, minimizing energy waste and interference.
To illustrate further, let’s outline the process in a structured table:
| Stage | Description | Key Components Involved | Potential Outcomes |
|---|---|---|---|
| Stimulus Detection | The cell senses an internal need, such as stress or growth requirement. | Sensors like transcription factors or metabolic enzymes. | Activation of gene expression for ligand production. |
| Ligand Synthesis | Production of the autocrine agent within the cell. | Ribosomes, endoplasmic reticulum. | Creation of hormones, cytokines, or growth factors. |
| Secretion | Release of the ligand into the extracellular environment. | Vesicles via exocytosis. | Ligand becomes available near the cell surface. |
| Receptor Binding | Ligand attaches to specific receptors on the same cell. | Autocrine receptors (e.g., receptor tyrosine kinases). | Conformational change in receptor structure. |
| Signal Transduction | Intracellular pathways relay the message. | Second messengers, kinases, phosphatases. | Amplification and transmission to target sites. |
| Cellular Response | Execution of the commanded function. | Nucleus for gene regulation, cytoskeleton for movement. | Changes like proliferation, differentiation, or apoptosis. |
| Termination | Deactivation to prevent continuous signaling. | Ligand degradation enzymes, receptor internalization. | Return to baseline state, ready for next cycle. |
This table captures the sequential nature, highlighting how each step builds on the previous for effective self-communication.
Examples of Autocrine Signaling in Action
Autocrine signaling manifests in numerous biological contexts, showcasing its versatility. Let’s explore several examples, starting with those in normal physiology and extending to pathological states.
In liver regeneration, following injury, hepatocytes release growth factors like hepatocyte growth factor (HGF) that bind back to their own receptors, stimulating division to restore tissue mass. This controlled proliferation ensures the liver doesn’t overgrow under normal conditions.
The immune system heavily relies on this signaling. T-lymphocytes, during activation, secrete interleukin-2 (IL-2), which binds to IL-2 receptors on the same cell, promoting clonal expansion. This amplifies the immune response against pathogens without needing constant input from other cells.
In wound healing, fibroblasts produce transforming growth factor-beta (TGF-β), which autocrinely stimulates collagen production, aiding tissue repair. This self-driven process accelerates closure of cuts or injuries.
Embryonic development offers another example: Stem cells in the early embryo use autocrine Wnt signaling to maintain pluripotency, deciding when to differentiate into specific lineages.
Even in the nervous system, neurons employ autocrine brain-derived neurotrophic factor (BDNF) to support survival and synaptic plasticity, enhancing learning and memory.
Pathologically, cancer cells exploit autocrine signaling for unchecked growth. Many tumors overproduce epidermal growth factor (EGF), binding to EGFR on the same cells, driving relentless division and metastasis.
In autoimmune diseases, dysregulated autocrine cytokine loops in immune cells can perpetuate inflammation, as seen in rheumatoid arthritis where TNF-alpha self-stimulates macrophages.
Plants also utilize similar mechanisms; for instance, root cells release auxins that autocrinely regulate elongation, adapting to soil conditions.
These examples underscore the signaling’s dual role: Beneficial in health, detrimental when hijacked.
For a comprehensive overview, here’s a detailed table of examples:
| Example Context | Signaling Molecule | Cell Type Involved | Function Performed | Biological Outcome |
|---|---|---|---|---|
| Liver Regeneration | Hepatocyte Growth Factor (HGF) | Hepatocytes | Stimulates cell division and migration. | Restores liver tissue after damage or surgery. |
| Immune Response Activation | Interleukin-2 (IL-2) | T-Lymphocytes | Promotes proliferation and differentiation. | Enhances defense against infections and cancers. |
| Wound Healing | Transforming Growth Factor-Beta (TGF-β) | Fibroblasts | Induces extracellular matrix production. | Facilitates scar formation and tissue repair. |
| Embryonic Stem Cell Maintenance | Wnt Proteins | Embryonic Stem Cells | Maintains pluripotency and self-renewal. | Supports proper organ formation during development. |
| Neuronal Survival | Brain-Derived Neurotrophic Factor (BDNF) | Neurons | Enhances synaptic strength and anti-apoptosis. | Improves neural plasticity and cognitive functions. |
| Cancer Cell Proliferation | Epidermal Growth Factor (EGF) | Tumor Cells (e.g., breast cancer) | Drives uncontrolled growth and invasion. | Leads to tumor expansion and potential metastasis. |
| Autoimmune Inflammation | Tumor Necrosis Factor-Alpha (TNF-α) | Macrophages | Sustains inflammatory cytokine release. | Contributes to chronic conditions like arthritis. |
| Plant Root Growth | Auxins | Root Meristem Cells | Regulates cell elongation and gravitropism. | Allows adaptation to environmental cues like gravity. |
| Platelet Aggregation | Thromboxane A2 | Platelets | Promotes clotting cascade activation. | Aids in hemostasis during vascular injury. |
| Bone Remodeling | Osteoprotegerin Ligand (OPGL) | Osteoclasts | Controls bone resorption rates. | Maintains skeletal integrity and calcium balance. |
This table illustrates the breadth of applications, from human physiology to plant biology, emphasizing its universal relevance.
Comparing Autocrine Signaling to Other Cell Signaling Types
To fully appreciate autocrine signaling, it’s helpful to contrast it with other pathways. Paracrine signaling involves signals affecting nearby cells, like neurotransmitters at synapses. Endocrine signaling sends hormones through blood to distant targets, as in insulin regulating glucose. Juxtacrine requires direct contact, such as Notch signaling in development.
Each type serves distinct purposes: Autocrine for self-control, paracrine for local coordination, endocrine for systemic regulation, and juxtacrine for precise neighbor interactions.
A comparison table clarifies these differences:
| Signaling Type | Target Distance | Key Examples | Transport Mechanism | Primary Role |
|---|---|---|---|---|
| Autocrine | Same cell | IL-2 in T-cells | Local diffusion | Self-regulation and amplification |
| Paracrine | Nearby cells | Nitric oxide in blood vessels | Short-range diffusion | Local tissue coordination |
| Endocrine | Distant cells | Thyroid hormones | Bloodstream circulation | Whole-body homeostasis |
| Juxtacrine | Adjacent cells via contact | Delta-Notch in neurogenesis | Membrane-bound ligands | Cell fate determination |
| Synaptic | Specific neurons | Acetylcholine at neuromuscular junctions | Vesicle release across synapse | Rapid neural communication |
This highlights how autocrine signaling fills a unique niche in the cellular communication repertoire.
Role of Autocrine Signaling in Diseases and Therapeutic Potential
Beyond normal functions, autocrine signaling is implicated in various diseases, offering targets for treatments. In cancer, autocrine loops fuel tumor autonomy; therapies like EGFR inhibitors disrupt these in lung cancer.
In infectious diseases, pathogens mimic autocrine signals to evade immunity, as some viruses induce self-suppressive cytokines in host cells.
Chronic inflammation disorders, like psoriasis, involve overactive autocrine growth factors in skin cells, leading to hyperproliferation.
Research explores harnessing this signaling for regenerative medicine, such as engineering cells to autocrinely produce factors for tissue engineering.
Future therapies might modulate autocrine pathways with small molecules or biologics, personalizing treatments based on individual signaling profiles.
Emerging Research and Insights on Autocrine Signaling
Recent studies reveal nuances, like how autocrine signals integrate with mechanosensing, where physical forces influence self-signaling in tissues.
In aging, diminished autocrine efficiency contributes to cellular senescence, suggesting interventions could extend healthspan.
Single-cell technologies uncover heterogeneity in autocrine responses, showing not all cells in a population signal identically.
These insights pave the way for advanced models of cellular behavior, blending computational biology with experiments.
Conclusion
Autocrine signaling embodies the ingenuity of biological systems, enabling cells to thrive through self-dialogue. From liver repair to cancer progression, its impacts are profound and multifaceted. As research advances, our grasp of this process will unlock new avenues for health innovations. By appreciating its intricacies, we better understand the symphony of life at the cellular level.
Frequently Asked Questions
FAQ 1: What distinguishes autocrine signaling from paracrine and endocrine signaling?
Autocrine signaling stands out in the world of cell communication because it allows a cell to essentially talk to itself, creating a self-sustaining loop that can fine-tune its own activities. In this process, a cell releases chemical messengers, such as growth factors or cytokines, which then bind directly to receptors on its own surface. This self-targeted approach is particularly useful for rapid, independent responses, like when a cell needs to quickly adjust to stress or initiate repair without waiting for input from others. Unlike broader signaling methods that involve multiple cells or distant organs, autocrine signaling keeps everything localized and personal to the originating cell.
Paracrine signaling, on the other hand, involves signals that travel short distances to affect nearby cells, fostering local coordination within tissues. For instance, in inflammation, cells might release substances that alert adjacent immune cells to join the response. Endocrine signaling takes this a step further by sending hormones through the bloodstream to reach far-off targets, regulating whole-body functions like metabolism or growth. These differences highlight how cells have evolved diverse strategies to communicate based on the scale and urgency of the need.
To better illustrate these distinctions, consider the following comparison table:
| Signaling Type | Target Cells | Distance Traveled | Key Messengers | Primary Functions | Examples in the Body |
|---|---|---|---|---|---|
| Autocrine | Same cell that produces the signal | Extremely short (local diffusion) | Cytokines, growth factors like IL-2 or EGF | Self-regulation, amplification of responses | T-cell proliferation during immune activation |
| Paracrine | Nearby cells | Short distances within tissues | Neurotransmitters, local hormones | Local coordination and tissue responses | Nitric oxide relaxing blood vessels in endothelium |
| Endocrine | Distant cells throughout the body | Long distances via bloodstream | Hormones like insulin or thyroid hormone | Systemic regulation and homeostasis | Insulin controlling blood sugar levels across organs |
This table underscores the unique efficiency of autocrine signaling in promoting cellular autonomy, while the others ensure collaborative efforts across larger scales.
FAQ 2: How does autocrine signaling play a role in cancer progression?
Autocrine signaling can unfortunately turn against the body when it goes awry, particularly in cancer, where cells exploit this self-communication to fuel uncontrolled growth. Normally, cells use autocrine loops to regulate their own division and survival in a balanced way, but in cancerous cells, genetic mutations can hijack these pathways. For example, tumor cells might overproduce growth factors that bind back to their own receptors, creating a perpetual “on” switch for proliferation. This self-stimulation allows cancers to become independent of external signals, making them more aggressive and harder to treat.
In many types of cancer, such as breast or lung cancer, the epidermal growth factor receptor (EGFR) pathway is a prime culprit. Cancer cells secrete ligands like EGF, which latch onto EGFR on the same cell, triggering cascades that promote cell division, invasion into surrounding tissues, and even resistance to chemotherapy. This autocrine overdrive not only accelerates tumor expansion but also contributes to metastasis, where cancer cells spread to new sites. Researchers have found that disrupting these loops with targeted drugs, like EGFR inhibitors, can slow disease progression, highlighting the pathway’s critical role.
Beyond growth, autocrine signaling in cancer influences the tumor microenvironment. Cancer cells might release cytokines that suppress immune detection, allowing them to evade the body’s defenses. In advanced stages, this self-signaling can lead to angiogenesis, where tumors signal themselves to form new blood vessels for nutrient supply. Overall, understanding these mechanisms has opened doors to personalized therapies that aim to break the vicious cycle of autocrine-driven malignancy, offering hope for better outcomes in patients.
FAQ 3: What is the significance of autocrine signaling in immune system function?
Autocrine signaling serves as a vital checkpoint in the immune system, enabling cells to self-regulate and amplify their responses during threats like infections or injuries. When an immune cell, such as a T-lymphocyte, encounters a pathogen, it doesn’t always rely solely on signals from other cells; instead, it can produce its own messengers to boost its activation. This self-boost ensures a swift and tailored defense, preventing delays that could allow invaders to gain ground.
Key aspects of its role include:
- Enhancing proliferation: Immune cells release cytokines like interleukin-2, which bind back to their receptors, prompting rapid division to increase their numbers for a stronger attack.
- Fine-tuning activation: This signaling helps cells adjust their intensity based on the threat level, avoiding overreactions that could lead to tissue damage.
- Supporting survival: In prolonged battles, autocrine factors promote cell longevity, ensuring sustained immunity without exhaustion.
- Coordinating with other pathways: It often integrates with paracrine signals from nearby cells, creating a layered defense network.
This mechanism’s importance becomes evident in conditions where it’s disrupted, such as in immunodeficiencies, where weakened autocrine loops impair the body’s ability to fight off diseases effectively.
FAQ 4: Can autocrine signaling influence heart health and disease?
In the context of heart health, autocrine signaling emerges as a double-edged sword, playing essential roles in maintaining cardiac function while also contributing to pathological changes during disease. Healthy heart cells, like cardiomyocytes, use this self-communication to respond to everyday stresses, such as fluctuations in workload or oxygen levels. For instance, they might secrete factors that bind to their own receptors, promoting minor adjustments in contraction strength or energy metabolism to keep the heart pumping efficiently.
However, in conditions like heart failure or after a myocardial infarction, autocrine signaling can drive harmful remodeling. Stressed cardiac cells release substances such as angiotensin II or endothelin-1, which act autocrinely to induce hypertrophy, where cells enlarge abnormally, or fibrosis, leading to stiffening of heart tissue. This remodeling initially compensates for damage but over time weakens the heart’s ability to pump blood, exacerbating failure. Studies show that these loops amplify inflammation and cell death, creating a cycle that progresses the disease.
Therapeutically, targeting autocrine pathways offers promise; drugs that block overactive receptors can mitigate remodeling, improving outcomes. By balancing this signaling, the heart can better adapt without tipping into dysfunction, underscoring its pivotal role in cardiovascular resilience.
FAQ 5: How do stem cells utilize autocrine signaling for their functions?
Stem cells harness autocrine signaling as a sophisticated tool for self-maintenance and decision-making, allowing them to remain versatile while responding to their environment. In embryonic stem cells, for example, this process involves releasing proteins like Wnt or leukemia inhibitory factor, which loop back to activate pathways that preserve their undifferentiated state. This self-sustaining communication ensures stem cells don’t prematurely specialize, keeping a reservoir ready for tissue development or repair.
As stem cells mature or face cues to differentiate, autocrine signals shift to guide these transitions. In adult stem cells, such as those in bone marrow, factors like transforming growth factor-beta help regulate quiescence or activation, balancing renewal with the body’s needs. This mechanism is crucial in regenerative processes, where injured tissues prompt stem cells to self-signal for proliferation and migration to the damage site.
Disruptions in this signaling can lead to issues like impaired healing or even stem cell-related cancers, where unchecked loops cause overgrowth. Overall, autocrine signaling empowers stem cells with autonomy, making them key players in growth, repair, and potential therapies for degenerative diseases.
FAQ 6: What are the primary molecules involved in autocrine signaling and their roles?
Autocrine signaling relies on a diverse array of molecules that act as messengers, receptors, and effectors, each contributing to the cell’s self-dialogue. These components work in concert to translate external or internal cues into actionable changes, from growth to survival.
To organize this, here’s a detailed table outlining key molecules:
| Molecule Type | Specific Examples | Role in Autocrine Signaling | Cellular Effects | Associated Processes |
|---|---|---|---|---|
| Ligands (Messengers) | Interleukin-2 (IL-2), Epidermal Growth Factor (EGF) | Bind to receptors on the same cell to initiate response | Triggers proliferation or differentiation | Immune activation, cancer growth |
| Receptors | IL-2 Receptor, EGFR (Epidermal Growth Factor Receptor) | Recognize and bind self-produced ligands | Activates intracellular pathways like JAK-STAT or MAPK | Signal transduction leading to gene expression changes |
| Second Messengers | Cyclic AMP (cAMP), Inositol Triphosphate (IP3) | Amplify the signal inside the cell | Regulates enzymes and ion channels | Metabolic adjustments and calcium release |
| Effector Proteins | Kinases (e.g., Protein Kinase A), Transcription Factors | Carry out the final commands | Alters protein function or DNA transcription | Cell cycle progression or apoptosis inhibition |
| Regulatory Molecules | Phosphatases, Inhibitory Proteins | Terminate or modulate the signal | Prevents overstimulation | Maintains balance to avoid chronic activation |
This framework shows how these molecules form a tightly regulated network essential for cellular precision.
FAQ 7: Why might autocrine signaling be considered a form of self-regulation in cells?
Autocrine signaling embodies the essence of cellular self-regulation by empowering cells to monitor and adjust their own states independently. In a bustling biological environment, cells can’t always depend on distant signals; this process allows them to secrete factors that provide immediate feedback, much like a thermostat sensing and correcting its own temperature. For vital functions like division or metabolism, this autonomy ensures efficiency and adaptability.
The importance lies in its ability to handle localized needs without broader systemic involvement. During development, cells use it to fine-tune growth rates, preventing imbalances. In mature tissues, it supports homeostasis, where cells self-correct minor disruptions. This self-reliance also conserves energy, as signals don’t travel far, and it minimizes interference from neighboring activities.
Ultimately, by fostering independence, autocrine signaling contributes to the resilience of multicellular organisms, allowing individual cells to thrive while contributing to the whole.
FAQ 8: In what ways does autocrine signaling contribute to autoimmune diseases?
Autocrine signaling can inadvertently fuel autoimmune diseases by perpetuating inflammatory cycles within immune cells. In conditions like rheumatoid arthritis, overactive autocrine loops cause cells to continuously stimulate themselves, leading to unchecked inflammation that attacks healthy tissues. For instance, macrophages might release tumor necrosis factor-alpha, which binds back to amplify cytokine production, creating a feedback loop that sustains joint damage.
This dysregulation often stems from genetic or environmental factors that alter receptor sensitivity or ligand production. In multiple sclerosis, T-cells use autocrine interleukin signals to enhance their attack on nerve sheaths, worsening demyelination. The result is a self-sustaining assault on the body, where the immune system fails to “turn off” appropriately.
Therapeutic strategies focus on interrupting these loops with biologics that block key cytokines, restoring balance and alleviating symptoms. Recognizing this role highlights how a normally protective mechanism can backfire in autoimmunity.
FAQ 9: How has research on autocrine signaling advanced our understanding of tissue repair?
Research into autocrine signaling has revolutionized our view of tissue repair, revealing how cells orchestrate their own healing without external orchestration. In wound healing, fibroblasts employ autocrine transforming growth factor-beta to stimulate collagen synthesis, accelerating scar formation and closure. This self-driven process ensures timely responses, especially in isolated or poorly vascularized areas.
Studies also show its integration in regenerative medicine, where enhancing autocrine pathways in stem cells could boost organ repair after injuries like strokes or liver damage. For example, hepatocytes use hepatocyte growth factor autocrinely to regenerate liver mass post-surgery, demonstrating innate recovery potential.
Ongoing investigations explore manipulating these signals for therapies, such as in chronic wounds where deficient autocrine activity delays healing. This knowledge paves the way for bioengineered solutions that mimic natural self-signaling to enhance recovery.
FAQ 10: What evolutionary advantages does autocrine signaling provide to organisms?
Autocrine signaling offers evolutionary advantages by promoting cellular independence, which likely originated in single-celled ancestors needing to self-regulate in unpredictable environments. This self-sufficiency allowed early life forms to adapt quickly to nutrients or threats, enhancing survival rates.
In multicellular evolution, it facilitated complex tissues by enabling cells to maintain local control amid growing interdependence. This balance reduced vulnerability; if one signaling pathway failed, autocrine loops could compensate, ensuring organismal robustness.
Moreover, it supported diversification, as cells could specialize while retaining self-management, leading to advanced systems like immune responses. Overall, this mechanism’s adaptability has been key to life’s progression from simple to sophisticated forms.
FAQ 11: How does autocrine signaling function within the nervous system?
Autocrine signaling plays a surprisingly vital role in the nervous system, where neurons and other cells use it to fine-tune their own activities and maintain synaptic health. In neurons, this self-communication often involves releasing neurotransmitters or growth factors that bind back to receptors on the same cell, helping to regulate everything from axon growth to synaptic plasticity. For example, during development, neurons might secrete brain-derived neurotrophic factor, which acts autocrinely to support their survival and strengthen connections, ensuring the brain wires itself correctly without solely relying on signals from distant cells.
This mechanism extends to more mature stages, where autocrine loops help neurons adapt to ongoing activity. In high-activity synapses, transient signals can trigger sustained autocrine responses, enhancing long-term potentiation, which is key for learning and memory. Disruptions in these loops have been linked to neurological issues, like impaired repair after injury or even contributions to disorders such as epilepsy, where unbalanced self-signaling might lead to excessive excitability.
Overall, autocrine signaling in the nervous system underscores how cells achieve independence in a highly interconnected environment, allowing precise, localized adjustments that complement broader neural networks.
FAQ 12: Does autocrine signaling occur in plants, and if so, how?
While autocrine signaling is often discussed in animal biology, it also appears in plants, where cells release molecules that influence their own growth and responses to the environment. In plants, this self-signaling helps coordinate processes like cell division, differentiation, and adaptation to stress, often through peptides or hormones that bind to nearby receptors on the same cell. For instance, during pollen tube growth, plants use autocrine mechanisms to maintain tube integrity over long distances, preventing bursting and ensuring successful reproduction.
To provide a clearer picture, here’s a structured table highlighting key examples and aspects of autocrine signaling in plants:
| Aspect | Description | Key Molecules Involved | Role in Plant Function | Examples |
|---|---|---|---|---|
| Pollen Tube Guidance | Plants secrete signals to regulate their own tube elongation during fertilization. | Rapid Alkalinization Factors (RALFs) like RALF4 and RALF19 | Maintains cell wall integrity and coordinates growth. | In flowering plants, autocrine loops prevent premature tube rupture. |
| Stomatal Development | Guard cells use self-signaling to control differentiation and spacing. | EPIDERMAL PATTERNING FACTOR 1 (EPF1) | Regulates stomatal density for efficient gas exchange. | Autocrine peptides ensure proper leaf patterning. |
| Root Growth Regulation | Root cells release auxins that act back on themselves. | Auxins and receptor kinases | Controls elongation and response to gravity or nutrients. | Helps roots adapt to soil conditions without external cues. |
| Stress Response | During drought or pathogen attack, cells signal themselves for defense. | Cytokinins and salicylic acid | Enhances resilience by modulating gene expression. | Autocrine cytokinin activity promotes cell survival under stress. |
| Embryonic Development | Early plant embryos use self-loops for patterning. | Wnt-like proteins | Supports symmetric division and tissue formation. | Ensures balanced growth in seedlings. |
This table shows how autocrine signaling adapts to plant needs, emphasizing its evolutionary conservation across kingdoms.
FAQ 13: What are the potential therapeutic targets in autocrine signaling pathways?
Therapeutic targeting of autocrine signaling holds promise for treating various diseases, particularly those involving uncontrolled cell growth or inflammation, by interrupting self-sustaining loops that drive pathology.
Key opportunities include:
- Cancer therapies: Blocking autocrine growth factor receptors, like EGFR in lung cancer, with inhibitors to halt tumor proliferation and metastasis.
- Cardiovascular treatments: Modulating autocrine factors in heart cells to reduce fibrosis and hypertrophy in heart failure, potentially improving cardiac function.
- Immune disorders: Targeting cytokine autocrine loops in autoimmune conditions to dampen excessive inflammation without broadly suppressing immunity.
- Liver diseases: Interfering with autocrine signals in hepatic stellate cells to prevent fibrosis in conditions like non-alcoholic steatohepatitis.
- Neurological applications: Enhancing autocrine neurotrophic factors to support neuron repair in neurodegenerative diseases.
These approaches aim to restore balance, offering more precise interventions with fewer side effects.
FAQ 14: Is autocrine signaling present in prokaryotes like bacteria?
Autocrine signaling isn’t exclusive to complex organisms; it also occurs in prokaryotes, such as bacteria, where it resembles quorum sensing but focuses on self-communication. In bacteria, cells secrete molecules called autoinducers that bind to their own receptors, allowing individual cells to sense their environment and adjust behaviors accordingly. This self-sensing helps bacteria respond to density or stress, even when alone, by triggering changes in gene expression for survival or virulence.
Unlike in eukaryotes, bacterial autocrine signaling often blends with community-wide communication, where the same molecules serve both individual and group purposes. For example, in some species, autoinducers promote biofilm formation on a single-cell level before escalating to collective action. This mechanism likely evolved early in life, providing prokaryotes with a way to adapt independently in fluctuating conditions.
Research suggests that understanding these prokaryotic loops could inform antibiotic development, as disrupting autocrine signals might weaken bacterial resilience without fostering resistance as quickly as traditional drugs.
FAQ 15: How does autocrine signaling interact with viral infections?
During viral infections, autocrine signaling becomes a battleground, where host cells use it to mount defenses while viruses exploit or suppress it to spread. Infected cells often release interferons that act autocrinely to enter an antiviral state, boosting their own resistance and limiting viral replication.
For a detailed overview, consider this table of interactions:
| Interaction Type | Description | Key Molecules | Host or Virus Benefit | Examples |
|---|---|---|---|---|
| Host Defense Activation | Cells signal themselves to upregulate antiviral genes. | Type I Interferons (e.g., IFN-alpha) | Enhances cellular immunity against spread. | In influenza, autocrine IFN creates a protective loop. |
| Viral Evasion | Viruses block autocrine receptors to prevent host response. | Viral proteins like NiV P | Allows unchecked replication by sequestering STAT proteins. | Nipah virus impairs IFN signaling. |
| Exosome Release | Infected cells use autocrine signals via exosomes to inhibit viruses. | Factors from liver endothelial cells in HCV | Reduces viral entry and spread. | Hepatitis C triggers autocrine IFN in endothelium. |
| Viral Promotion | Some viruses induce autocrine loops to enhance host cell survival for replication. | v-Chemokines in poxviruses | Prolongs cell life for virus production. | Promotes paracrine effects too, aiding infection. |
| Microbiota Influence | Gut bacteria modulate autocrine IFN via microbiota signals. | Commensal-derived factors | Strengthens barrier against enteric viruses. | Protects against rotavirus or norovirus. |
This illustrates the dynamic tug-of-war in infections, highlighting potential antiviral strategies.
FAQ 16: What is the evolutionary origin of autocrine signaling?
The evolution of autocrine signaling likely traces back to early single-celled organisms, where self-communication provided a survival edge in harsh environments by allowing cells to self-regulate without partners.
Insights into its development include:
- Prokaryotic roots: In bacteria, primitive autocrine-like quorum sensing enabled individual adaptation, evolving into more complex loops in eukaryotes.
- Transition to multicellularity: As organisms became multicellular, autocrine signaling supported cell autonomy, complementing new paracrine and endocrine systems.
- Conservation across kingdoms: Shared mechanisms in plants and animals suggest ancient origins, with peptides and receptors diverging for specialized roles.
- Role in development: It facilitated embryonic patterning, ensuring cells could self-guide differentiation in evolving body plans.
- Pathological adaptations: In diseases like cancer, hijacked autocrine loops mimic ancient growth strategies, revealing evolutionary trade-offs.
This pathway’s persistence underscores its fundamental importance in life’s progression.
FAQ 17: In what ways does autocrine signaling relate to metabolic disorders?
Autocrine signaling intersects with metabolic disorders by influencing how cells handle energy, lipids, and hormones, often exacerbating conditions when dysregulated. In obesity or diabetes, adipose cells might overproduce autocrine factors that promote inflammation and insulin resistance, creating a vicious cycle where self-signaling amplifies fat accumulation and metabolic imbalance.
For instance, in liver-related metabolic issues, autocrine loops in hepatocytes can drive steatosis by mismanaging lipid synthesis, contributing to non-alcoholic fatty liver disease. Similarly, in pancreatic beta cells, disrupted autocrine insulin signaling impairs glucose regulation, worsening type 2 diabetes.
Targeting these pathways could offer new treatments, like modulating autocrine cytokines to restore metabolic harmony and prevent progression to severe complications.
FAQ 18: What methods are used to study autocrine signaling?
Studying autocrine signaling requires specialized techniques to capture its self-contained nature, often combining cellular, molecular, and computational approaches.
Here’s a table outlining primary methods:
| Method | Description | Tools/Techniques | Advantages | Limitations | Applications |
|---|---|---|---|---|---|
| Cell Patterning | Isolates cells to modulate autocrine cues without altering other factors. | Microfluidics and patterning devices | Allows precise control of diffusive signals. | Technically complex setup. | Cancer cell growth studies. |
| Microphysiometry | Measures real-time ligand binding and cellular responses. | Biosensors for pH or metabolism changes | Quantifies dynamic autocrine activity. | Limited to certain cell types. | Ligand-receptor interactions. |
| Computational Modeling | Simulates signal diffusion and receptor binding. | Mathematical models of spatial range | Predicts loop behaviors in tissues. | Relies on accurate parameters. | Epidermal growth factor studies. |
| Single-Cell Sequencing | Analyzes gene expression in individual cells for signaling patterns. | RNA-seq and spatial transcriptomics | Reveals heterogeneity in loops. | High cost and data complexity. | Immune cell autocrine cytokines. |
| Antibody-Based Selection | Uses combinatorial libraries to identify agonists or inhibitors. | Intracellular antibody screening | Generates tools for pathway manipulation. | Requires advanced libraries. | Stem cell differentiation. |
These methods help unravel the intricacies of autocrine processes.
FAQ 19: How is autocrine signaling involved in the aging process?
Autocrine signaling contributes to aging by influencing cellular senescence and tissue maintenance, where shifts in self-communication can accelerate decline or promote longevity.
Notable involvements are:
- Senescent cell reinforcement: Aging cells use autocrine loops, like BDNF-TrkB, to sustain their survival, spreading senescence-associated secretory phenotype factors that inflame nearby tissues.
- Decline in protective signals: Reduced autocrine pituitary adenylate cyclase-activating polypeptide impairs endothelial function, contributing to vascular aging.
- Podocyte dysfunction: In kidneys, autocrine changes in aging podocytes lead to glomerular issues, highlighting organ-specific effects.
- Stem cell regulation: Autocrine Wnt or growth factors diminish, affecting regeneration and accelerating tissue wear.
- Therapeutic implications: Boosting beneficial autocrine signals might mitigate age-related diseases, extending healthy lifespan.
This reveals autocrine signaling as a key modulator in aging biology.
FAQ 20: What directions might future research on autocrine signaling take?
Future research on autocrine signaling is poised to explore its untapped potential in precision medicine, focusing on dynamic mechanisms that could revolutionize treatments for chronic diseases. Scientists are increasingly interested in how autocrine loops interact with allosteric regulation, like in TGF-beta pathways, where understanding conformational changes might lead to novel inhibitors that selectively disrupt harmful signals without affecting beneficial ones.
Another promising area involves integrating autocrine studies with single-cell technologies and AI modeling to map heterogeneous signaling in tissues, revealing personalized disease patterns in cancer or fibrosis. In regenerative medicine, enhancing autocrine factors could improve stem cell therapies, promoting self-repair in damaged organs.
Overall, as tools advance, research will likely shift toward real-time imaging and targeted modulation, uncovering evolutionary insights and new therapeutic avenues for conditions driven by dysregulated self-communication.
Acknowledgement
The Examsmeta.com website would like to expresses its gratitude to the wealth of scientific resources that made this article, “Autocrine Signaling: Mechanisms, Roles, and Therapeutic Potential,” possible. The insights drawn from cutting-edge research and detailed explanations provided by reputable platforms have been instrumental in shaping a clear and comprehensive narrative. Specifically, acknowledges the contributions of Nature (www.nature.com), ScienceDirect (www.sciencedirect.com), PubMed (pubmed.ncbi.nlm.nih.gov), and Cell (www.cell.com) for their authoritative publications and accessible databases. These sources offered critical information on cellular mechanisms, signaling pathways, and their implications across health and disease.

