Beta oxidation is a fundamental metabolic process that allows our bodies to convert stored fats into usable energy. This catabolic pathway breaks down fatty acid molecules, primarily in the mitochondria of eukaryotic cells, generating acetyl-CoA that fuels the citric acid cycle and ultimately produces ATP through the electron transport chain. Named for the oxidation occurring at the beta carbon of the fatty acid chain, beta oxidation plays a crucial role in energy production, especially during fasting, exercise, or when carbohydrate supplies are low.
In prokaryotes, this process happens in the cytosol, highlighting evolutionary differences in cellular organization. Understanding beta oxidation not only sheds light on basic biochemistry but also explains why fats are such an efficient energy source, providing about twice the energy per gram compared to carbohydrates or proteins.
Table of Contents
In everyday life, think about how your body switches to burning fat after a long workout or overnight fast. During these times, beta oxidation ramps up to meet energy demands, contributing up to 80 percent of the body’s energy needs in prolonged fasting states. This process is tightly regulated to prevent unnecessary breakdown when other fuels are available, ensuring metabolic efficiency. For instance, in athletes, enhanced beta oxidation capacity can improve endurance by sparing glycogen stores. However, disruptions in this pathway can lead to serious metabolic disorders, underscoring its importance in health and disease.
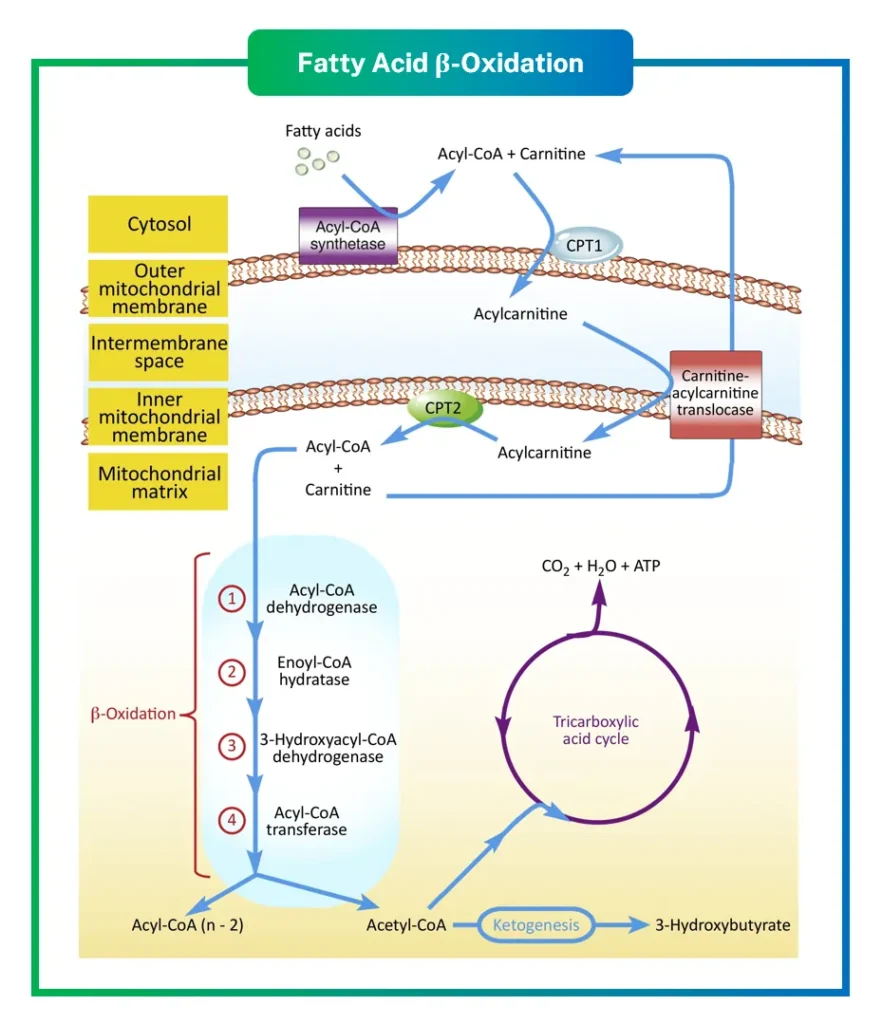
The Basics of Fatty Acid Activation and Transport
Before beta oxidation can begin, fatty acids must be activated and transported into the mitochondria, where the magic happens. Free fatty acids, released from adipose tissue through lipolysis, carry a negative charge that prevents them from crossing biological membranes easily. Lipolysis itself is triggered by hormones like epinephrine in response to low insulin levels, activating hormone-sensitive lipase to cleave fatty acids from triglycerides.
Once in the bloodstream, these fatty acids bind to plasma albumin for transport to target cells. Upon arrival, they cross the cell membrane via specific transporters, such as those from the SLC27 family. Inside the cytosol, activation occurs through the action of long-chain-fatty-acid-CoA ligase, also known as acyl-CoA synthetase. This enzyme catalyzes a two-step reaction: first, the fatty acid reacts with ATP to form a fatty acyl adenylate and pyrophosphate; then, this intermediate reacts with coenzyme A (CoA) to produce fatty acyl-CoA and AMP.
For long-chain fatty acids, which are the most common in diets, entry into the mitochondrial matrix requires the carnitine shuttle system. This shuttle is essential because acyl-CoA cannot directly penetrate the inner mitochondrial membrane. The process involves several key enzymes and steps:
- Carnitine palmitoyltransferase I (CPT-I), located on the outer mitochondrial membrane, transfers the acyl group from acyl-CoA to carnitine, forming acyl-carnitine.
- A translocase protein then shuttles acyl-carnitine across the inner membrane in exchange for free carnitine moving out.
- Inside the matrix, carnitine palmitoyltransferase II (CPT-II) converts acyl-carnitine back to acyl-CoA, releasing carnitine to be recycled.
Short-chain fatty acids, however, can diffuse directly into the mitochondria without this shuttle, simplifying their processing. This distinction ensures efficient handling of various fatty acid lengths. Interestingly, very long-chain fatty acids (over 20 carbons) are initially oxidized in peroxisomes before being shortened for mitochondrial beta oxidation, adding another layer of compartmentalization in eukaryotic cells.
Detailed Mechanism of Beta Oxidation
Once inside the mitochondrial matrix, the actual beta oxidation cycle commences, systematically cleaving two-carbon units from the fatty acid chain. Each cycle shortens the chain by two carbons, producing acetyl-CoA, which enters the citric acid cycle (also known as the TCA cycle or Krebs cycle). The overall reaction for one cycle of beta oxidation can be represented as:
$$C_n\text{-acyl-CoA} + \text{FAD} + \text{NAD}^+ + \text{H}2\text{O} + \text{CoA} \rightarrow$$ $$ C{n-2}\text{-acyl-CoA} + \text{FADH}_2 + \text{NADH} + \text{H}^+ $$ $$ + \text{acetyl-CoA}$$
This equation highlights the involvement of electron carriers FAD and NAD+, which are reduced to FADH2 and NADH, respectively. These reduced forms then donate electrons to the electron transport chain for ATP synthesis.
The cycle consists of four enzymatic steps, repeated until the entire fatty acid is degraded. Primarily facilitated by the mitochondrial trifunctional protein complex associated with the inner membrane, these steps ensure efficient processing. Let’s break them down:
- Dehydrogenation: The first step involves the removal of two hydrogen atoms from the alpha and beta carbons, creating a trans double bond. Catalyzed by acyl-CoA dehydrogenase, this produces trans-delta-2-enoyl-CoA and reduces FAD to FADH2. Different isoforms of this enzyme handle short, medium, long, and very long-chain fatty acids, allowing specificity.
- Hydration: Next, enoyl-CoA hydratase adds water across the double bond, forming L-3-hydroxyacyl-CoA. This step is stereospecific, ensuring the correct configuration for subsequent reactions.
- Second Dehydrogenation: 3-Hydroxyacyl-CoA dehydrogenase oxidizes the hydroxyl group at the beta carbon, producing 3-ketoacyl-CoA and reducing NAD+ to NADH. This enzyme uses NAD+ as its cofactor, linking the process to the respiratory chain.
- Thiolysis: Finally, thiolase (or beta-ketothiolase) cleaves the bond between the alpha and beta carbons using CoA, releasing acetyl-CoA and a shortened acyl-CoA ready for another cycle.
For even-chain fatty acids, this results in complete conversion to acetyl-CoA units. Odd-chain fatty acids, however, yield a final propionyl-CoA, which is converted to succinyl-CoA via a vitamin B12-dependent pathway, integrating into the TCA cycle differently.
Energy Yield from Beta Oxidation
One of the most fascinating aspects of beta oxidation is its high energy efficiency. For a typical fatty acid like palmitic acid (16 carbons), seven cycles occur, producing eight acetyl-CoA molecules. Each cycle generates one FADH2 and one NADH, while each acetyl-CoA yields about 10 ATP through the TCA cycle and oxidative phosphorylation.
To calculate the total ATP yield, consider:
- Activation cost: 2 ATP equivalents (since AMP is produced, equivalent to 2 ATP).
- Per cycle: 4 ATP from FADH2 (1.5 ATP) and NADH (2.5 ATP), but actually, modern estimates use 1.5 for FADH2 and 2.5 for NADH.
- From acetyl-CoA: Each produces 3 NADH (7.5 ATP), 1 FADH2 (1.5 ATP), and 1 GTP (1 ATP), totaling 10 ATP.
For palmitic acid:
- Cycles: 7, yielding 7 FADH2 (10.5 ATP) and 7 NADH (17.5 ATP).
- Acetyl-CoA: 8, yielding 80 ATP.
- Total gross: 108 ATP; net: 106 ATP after activation.
This makes fats an superior energy store, as oxidizing one gram of fat yields about 9 kcal, compared to 4 kcal for carbohydrates.
| Fatty Acid Example | Chain Length | Number of Cycles | Acetyl-CoA Produced | Net ATP Yield |
|---|---|---|---|---|
| Butyric Acid | 4 carbons | 1 | 2 | 15 |
| Caprylic Acid | 8 carbons | 3 | 4 | 41 |
| Palmitic Acid | 16 carbons | 7 | 8 | 106 |
| Stearic Acid | 18 carbons | 8 | 9 | 120 |
| Arachidic Acid | 20 carbons | 9 | 10 | 134 |
This table illustrates how longer chains provide more energy, emphasizing why dietary fats are calorie-dense.
Regulation of Beta Oxidation
Beta oxidation is not always active; it’s finely tuned to the body’s needs. Key regulatory points include:
- Hormonal Control: Insulin inhibits lipolysis, reducing fatty acid availability, while glucagon and epinephrine promote it during fasting or stress.
- CPT-I Inhibition: Malonyl-CoA, produced by acetyl-CoA carboxylase during fatty acid synthesis, inhibits CPT-I, preventing simultaneous synthesis and breakdown. This “metabolic switch” ensures efficiency.
- Enzyme Expression: In high-energy demand tissues like muscle and heart, genes for beta oxidation enzymes are upregulated via transcription factors like PPAR-alpha.
- Feedback Inhibition: High NADH/NAD+ ratios from the TCA cycle can slow dehydrogenase steps.
During exercise, increased AMP activates AMPK, which indirectly boosts beta oxidation by reducing malonyl-CoA levels. In contrast, in fed states, high insulin promotes storage over breakdown.
Special Cases and Variations
While mitochondrial beta oxidation handles most fatty acids, peroxisomes oxidize very long-chain and branched-chain ones, like those from phytanic acid in dairy. Peroxisomal oxidation doesn’t produce ATP directly; instead, it shortens chains for mitochondrial completion, with FADH2 oxidizing to hydrogen peroxide, detoxified by catalase.
In plants and some microbes, beta oxidation occurs in glyoxysomes, linking to gluconeogenesis. For unsaturated fatty acids, additional enzymes like isomerases and reductases handle double bonds.
Odd-chain fatty acids, rare in diets but from some plants, end with propionyl-CoA, converted to methylmalonyl-CoA then succinyl-CoA, requiring biotin and vitamin B12.
| Aspect | Mitochondrial Beta Oxidation | Peroxisomal Beta Oxidation |
|---|---|---|
| Location | Mitochondrial matrix | Peroxisomes |
| Chain Length Preference | Short to long (up to 20C) | Very long (>20C), branched |
| Energy Production | Generates NADH, FADH2 for ATP | Produces H2O2, no direct ATP |
| Key Enzymes | Trifunctional protein | Separate enzymes |
| End Products | Acetyl-CoA | Shortened acyl-CoA for mitochondria |
This comparison shows compartmentalization’s role in handling diverse fatty acids.
Clinical Significance and Disorders
Defects in beta oxidation lead to fatty acid oxidation disorders (FAODs), inherited conditions affecting energy production. For example, medium-chain acyl-CoA dehydrogenase (MCAD) deficiency, the most common, causes hypoglycemia and lethargy during fasting, as medium-chain fats accumulate.
Very long-chain acyl-CoA dehydrogenase (VLCAD) deficiency impacts heart and muscle, leading to cardiomyopathy. Treatment involves avoiding fasting, medium-chain triglyceride supplements, and carnitine therapy.
In diabetes, uncontrolled beta oxidation contributes to ketoacidosis from excess acetyl-CoA forming ketones. Conversely, in obesity, impaired beta oxidation may promote fat accumulation.
Research shows enhancing beta oxidation could treat metabolic syndromes, with drugs targeting PPARs to boost enzyme activity.
Beta Oxidation in Different Tissues
Most tissues oxidize fatty acids, but exceptions exist. Red blood cells lack mitochondria, relying on glucose via glycolysis. Brain neurons prefer glucose or ketones, as fatty acids don’t cross the blood-brain barrier easily, though astrocytes can perform beta oxidation to support neurons.
In the liver, beta oxidation fuels ketogenesis during starvation, producing ketones for other organs. Heart muscle derives 60-90 percent of energy from fats, making beta oxidation vital for cardiac function.
During pregnancy, placental beta oxidation supports fetal energy needs, highlighting its role in development.
Evolutionary and Comparative Insights
From an evolutionary perspective, beta oxidation’s conservation across species underscores its efficiency. In bacteria, it’s cytosolic, integrated with other pathways. In humans, mitochondrial localization couples it to oxidative phosphorylation, maximizing ATP yield.
Comparative studies show hibernating animals upregulate beta oxidation genes to survive on fat stores, offering insights into human fasting adaptations.
In summary, beta oxidation is a powerhouse process, turning dietary and stored fats into life-sustaining energy. Its intricate steps, regulation, and variations make it a cornerstone of metabolism, with implications for health, nutrition, and performance. Whether you’re an athlete pushing limits or someone managing metabolic health, appreciating this pathway reveals the body’s remarkable efficiency in fuel utilization.
Frequently Asked Questions
FAQ 1: What Is Beta Oxidation of Fatty Acids and Why Is It Important?
Beta oxidation is a key metabolic process where your body breaks down fatty acids to produce energy. This happens mainly in the mitochondria of cells in animals and humans, but in bacteria, it occurs in the cytosol. Essentially, it chops up long chains of fatty acids into smaller pieces, specifically two-carbon units called acetyl-CoA, which then feed into other cycles to generate ATP, the energy currency of cells. The name comes from the oxidation happening at the beta carbon position in the fatty acid chain, turning it into a carbonyl group and setting up for the next round.
This process is vital because fats are a dense energy source, providing more than twice the calories per gram compared to carbs or proteins. During times like fasting, intense exercise, or low-carb diets, your body relies heavily on beta oxidation to keep things running. For example, it can supply up to 80 percent of energy needs in prolonged fasting. Without it, you’d struggle to tap into fat stores, leading to fatigue or metabolic issues. It’s also linked to overall health; efficient beta oxidation helps maintain weight, supports heart function, and even influences how the body handles conditions like diabetes. Research shows that enhancing this pathway through lifestyle changes can boost endurance in athletes by preserving glycogen.
Beyond energy, beta oxidation plays roles in detoxification and hormone production. In the liver, it contributes to ketone body formation, which the brain uses as an alternative fuel when glucose is scarce. Disruptions here can cause serious problems, like buildup of toxic fatty acids. Overall, understanding beta oxidation reveals how our bodies efficiently switch fuels, adapting to daily demands and ensuring survival in varying nutritional states.
FAQ 2: How Does Beta Oxidation Work Step by Step?
Beta oxidation unfolds in a series of precise steps inside the mitochondria, transforming fatty acids into usable energy components. It starts after fatty acids are activated and transported into the mitochondrial matrix. The cycle repeats, shortening the chain by two carbons each time until it’s fully broken down.
To break it down clearly:
- Activation: Fatty acids are first converted to acyl-CoA in the cytosol using ATP and coenzyme A, catalyzed by acyl-CoA synthetase. This step prepares them for transport.
- Transport via Carnitine Shuttle: For longer chains, acyl-CoA is transferred to carnitine by CPT-I on the outer mitochondrial membrane, shuttled across, and reconverted by CPT-II inside.
- Dehydrogenation: Once inside, acyl-CoA dehydrogenase removes hydrogens from carbons 2 and 3, forming a trans double bond and producing FADH2.
- Hydration: Enoyl-CoA hydratase adds water to the double bond, creating L-3-hydroxyacyl-CoA.
- Oxidation: 3-Hydroxyacyl-CoA dehydrogenase oxidizes the hydroxy group, yielding 3-ketoacyl-CoA and NADH.
- Thiolysis: Thiolase cleaves the chain, releasing acetyl-CoA and a shortened acyl-CoA for the next cycle.
Each cycle’s overall reaction is $$C_n\text{-acyl-CoA} + \text{FAD} + \text{NAD}^+ + \text{H}2\text{O} + \text{CoA} \rightarrow$$ $$C{n-2}\text{-acyl-CoA} + \text{FADH}_2 + \text{NADH} + \text{H}^+ + \text{acetyl-CoA}$$. The acetyl-CoA enters the citric acid cycle, while NADH and FADH2 fuel the electron transport chain for ATP. This meticulous process ensures fats are efficiently converted, with variations for unsaturated or branched chains requiring extra enzymes.
FAQ 3: What Role Does the Carnitine Shuttle Play in Beta Oxidation?
The carnitine shuttle is like a ferry system that gets fatty acids across the mitochondrial membrane so beta oxidation can happen. Free fatty acids can’t just slip through because of their charge, so after activation to acyl-CoA in the cytosol, long-chain ones need this shuttle. It involves carnitine, a molecule derived from amino acids, acting as a carrier.
First, carnitine palmitoyltransferase I on the outer membrane swaps the CoA for carnitine, making acyl-carnitine. This crosses the inner membrane via a translocase, exchanging with free carnitine going out. Then, inside the matrix, carnitine palmitoyltransferase II switches it back to acyl-CoA, freeing carnitine to recycle. Short chains skip this, diffusing directly.
This mechanism prevents fatty acid buildup and regulates metabolism. High malonyl-CoA levels, from carb-rich states, inhibit CPT-I, halting the shuttle and favoring fat storage over breakdown. In conditions like carnitine deficiency, the shuttle fails, leading to energy shortages. Supplements like L-carnitine are sometimes used to boost this system, especially in athletes or those with metabolic disorders, though evidence varies. Overall, the shuttle ensures beta oxidation runs smoothly, linking cytosolic activation to mitochondrial action.
FAQ 4: How Much ATP Is Produced from Beta Oxidation?
Beta oxidation is incredibly efficient at generating ATP from fats, far outpacing glucose breakdown. The yield depends on the fatty acid’s length, with each cycle producing energy carriers that lead to ATP via oxidative phosphorylation. For instance, palmitic acid (16 carbons) undergoes 7 cycles, yielding 8 acetyl-CoA, 7 NADH, and 7 FADH2, minus 2 ATP for activation, netting around 106 ATP.
| Fatty Acid | Carbon Atoms | Cycles | Acetyl-CoA | NADH | FADH2 | Net ATP Yield |
|---|---|---|---|---|---|---|
| Butyric Acid | 4 | 1 | 2 | 1 | 1 | 15 |
| Caproic Acid | 6 | 2 | 3 | 2 | 2 | 29 |
| Lauric Acid | 12 | 5 | 6 | 5 | 5 | 77 |
| Palmitic Acid | 16 | 7 | 8 | 7 | 7 | 106 |
| Stearic Acid | 18 | 8 | 9 | 8 | 8 | 120 |
| Oleic Acid (unsaturated) | 18 | 8 (with adjustments) | 9 | 7 | 7 | ~118 (less due to extra steps) |
| Arachidonic Acid | 20 | 9 | 10 | 9 | 9 | 134 |
These calculations use modern estimates: 2.5 ATP per NADH and 1.5 per FADH2, plus 10 ATP per acetyl-CoA from the TCA cycle. Unsaturated fats yield slightly less due to skipped dehydrogenation steps. This high output explains why fats are preferred for long-term energy storage.
FAQ 5: What Regulates Beta Oxidation in the Body?
Regulation of beta oxidation keeps energy production in check, preventing waste or overload. It’s influenced by hormones, energy status, and metabolites, ensuring it ramps up when needed, like during fasting.
Key regulators include:
- Hormonal Signals: Glucagon and epinephrine boost lipolysis, increasing fatty acid supply, while insulin suppresses it, favoring storage.
- Malonyl-CoA Inhibition: This molecule, high during fed states, blocks CPT-I, stopping the carnitine shuttle and thus beta oxidation.
- Energy Sensors: AMPK, activated by low ATP, reduces malonyl-CoA and promotes oxidation; high NADH levels slow dehydrogenase enzymes.
- Transcriptional Control: PPAR-alpha upregulates beta oxidation genes in response to fatty acids or fasting.
- Cofactor Availability: NAD+ and FAD levels affect reaction rates, linking to overall cellular redox state.
This multi-level control integrates beta oxidation with other pathways, like preventing simultaneous fat synthesis and breakdown for efficiency.
FAQ 6: What Are Fatty Acid Oxidation Disorders and Their Symptoms?
Fatty acid oxidation disorders are rare genetic conditions where enzymes for breaking down fats are missing or faulty, leading to energy deficits and toxic buildups. These autosomal recessive disorders affect mitochondrial beta oxidation, causing the body to struggle during fasting or illness when fat reliance increases.
Common types include MCAD deficiency, the most frequent, where medium-chain fats can’t be processed, risking sudden hypoglycemia or lethargy in infants. VLCAD deficiency hits longer chains, potentially causing heart muscle weakness or rhabdomyolysis. Symptoms often appear in early childhood: vomiting, seizures, coma during infections, or muscle pain in adults. Without treatment, they can be fatal, but newborn screening catches many.
Management involves avoiding fasting, using medium-chain triglycerides for energy, and carnitine supplements. Research into gene therapies offers hope. These disorders highlight beta oxidation’s critical role, as even mild cases can worsen with stress.
FAQ 7: What Are the Key Differences Between Mitochondrial and Peroxisomal Beta Oxidation?
Mitochondrial and peroxisomal beta oxidation both break down fatty acids but differ in location, substrates, and outcomes, complementing each other for complete metabolism.
| Feature | Mitochondrial Beta Oxidation | Peroxisomal Beta Oxidation |
|---|---|---|
| Location | Mitochondrial matrix | Peroxisomes |
| Preferred Substrates | Short to long-chain fatty acids (up to 20 carbons) | Very long-chain (>20 carbons) and branched-chain fatty acids |
| Energy Output | Produces NADH and FADH2 for ATP via electron transport chain | Generates H2O2 (detoxified by catalase), no direct ATP |
| Enzymes | Trifunctional protein complex handles multiple steps | Separate enzymes for each step; first dehydrogenase uses O2 directly |
| Chain Shortening | Complete breakdown to acetyl-CoA | Partial shortening, products sent to mitochondria |
| Regulation | Tightly linked to energy needs, inhibited by malonyl-CoA | Induced by high fatty acid loads, less ATP-coupled |
| Key Differences in Process | NADH from third step reoxidized for ATP; more efficient | No NADH reoxidation in chain; produces heat from H2O2 |
These distinctions ensure diverse fatty acids are handled efficiently, with peroxisomes acting as a first line for tough chains.
FAQ 8: How Does Beta Oxidation Differ for Even-Chain and Odd-Chain Fatty Acids?
Even-chain fatty acids, common in diets like palmitic acid, go through beta oxidation to yield only acetyl-CoA units. Each cycle removes two carbons, fully converting the chain into these two-carbon pieces that enter the citric acid cycle for energy.
Odd-chain fatty acids, rarer but found in some plants or dairy, end differently. After cycles, they leave a three-carbon propionyl-CoA. This converts to D-methylmalonyl-CoA via propionyl-CoA carboxylase (needing biotin), then to L-methylmalonyl-CoA by epimerase, and finally to succinyl-CoA by methylmalonyl-CoA mutase (requiring vitamin B12). Succinyl-CoA joins the TCA cycle, potentially feeding gluconeogenesis.
This difference means odd chains can contribute to glucose production, unlike even chains focused on ketones or direct energy. Deficiencies in these enzymes cause disorders like propionic acidemia, with symptoms including metabolic acidosis.
FAQ 9: In Which Tissues Does Beta Oxidation Primarily Occur?
Beta oxidation happens in various tissues, tailored to their energy needs, though not all cells use it equally. It’s most active where high energy demand meets fat availability.
Notable tissues include:
- Liver: Major site, producing ketones from excess acetyl-CoA for export to other organs during starvation.
- Heart Muscle: Derives 60-90% of energy from fats, relying on beta oxidation for constant pumping.
- Skeletal Muscle: Increases during exercise, sparing glucose for bursts.
- Kidneys: Uses it for reabsorption work.
Exceptions: Brain neurons avoid it, preferring glucose or ketones; red blood cells lack mitochondria entirely. Astrocytes in the brain can perform it to support neurons indirectly.
FAQ 10: How Is Beta Oxidation Linked to Ketogenesis?
Beta oxidation and ketogenesis are closely tied, especially in the liver during low-carb states. When beta oxidation produces excess acetyl-CoA beyond TCA cycle capacity, it shifts to ketone production.
This starts with two acetyl-CoA forming acetoacetyl-CoA via thiolase, then HMG-CoA synthase adds another to make HMG-CoA. HMG-CoA lyase cleaves it into acetoacetate, which reduces to beta-hydroxybutyrate or decarboxylates to acetone. These ketones fuel the brain and muscles, sparing glucose.
The link ensures survival in fasting; without it, hypoglycemia could occur. In diabetes, uncontrolled beta oxidation floods ketones, causing ketoacidosis. Diets like keto leverage this for weight loss, boosting fat burn.
FAQ 11: How Does Beta Oxidation Process Unsaturated Fatty Acids?
Beta oxidation typically handles saturated fatty acids by systematically removing two-carbon units, but unsaturated fatty acids, which have double bonds, require additional steps to navigate those bonds. These fats, common in diets from sources like olive oil or fish, enter the pathway similarly but need extra enzymes to adjust the double bond positions for the standard cycle to continue. For instance, if a double bond is in the wrong spot, an isomerase shifts it to the trans configuration at the delta-2 position, allowing dehydrogenation to proceed.
In monounsaturated fatty acids like oleic acid, the process skips one dehydrogenation step because the double bond already exists, slightly reducing the energy yield compared to saturated counterparts. Polyunsaturated fatty acids, such as linoleic acid, might require a reductase to convert conjugated double bonds into a form compatible with hydration. This adaptation ensures efficient breakdown, though it can produce unique intermediates that influence cellular signaling or inflammation.
Overall, these modifications highlight the pathway’s flexibility, evolved to handle diverse dietary fats. Without them, unsaturated fats could accumulate, potentially leading to metabolic imbalances. Studies show that diets rich in unsaturated fats can enhance mitochondrial function, supporting better energy production during beta oxidation. This is particularly relevant in heart tissue, where unsaturated fatty acid oxidation contributes significantly to ATP supply.
FAQ 12: What Role Does Beta Oxidation Play in Weight Loss?
Beta oxidation is central to weight loss because it breaks down stored fats into energy, effectively reducing body fat when calorie intake is lower than expenditure. During dieting or fasting, the body shifts from using carbs to fats, ramping up this process in muscles and the liver to burn triglycerides mobilized from adipose tissue. This not only provides sustained energy but also prevents muscle breakdown by sparing proteins.
However, simply increasing beta oxidation doesn’t guarantee fat loss; it must align with overall energy balance. High-fat diets can sometimes hinder it by overwhelming the system, leading to fat storage instead. Exercise combined with dietary strategies, like intermittent fasting, boosts enzyme activity in the pathway, enhancing fat utilization. For example, in obese individuals, improved beta oxidation correlates with better weight maintenance post-loss.
Interestingly, certain compounds can amplify this effect, but the key is consistency in lifestyle changes. Over time, as beta oxidation efficiency improves, it contributes to metabolic health, reducing risks like insulin resistance that often accompany weight regain.
FAQ 13: How Does Beta Oxidation Compare to Glycolysis in Energy Production?
Beta oxidation and glycolysis are both crucial for ATP generation but differ in substrates, locations, and efficiency. Glycolysis breaks down glucose in the cytosol, quickly producing 2 ATP per glucose via substrate-level phosphorylation, ideal for short bursts of energy. In contrast, beta oxidation occurs in mitochondria, oxidizing fatty acids to yield far more ATP—up to 106 for a 16-carbon fat—through oxidative phosphorylation, suiting prolonged activities.
| Aspect | Beta Oxidation | Glycolysis |
|---|---|---|
| Substrate | Fatty acids from fats | Glucose from carbs |
| Location | Mitochondria (eukaryotes) | Cytosol |
| ATP Yield Example | ~106 ATP from palmitate | 2 ATP net from glucose (anaerobic); up to 32 with oxygen |
| Speed | Slower, sustained | Fast, for quick energy |
| Oxygen Requirement | Aerobic, produces NADH/FADH2 | Can be anaerobic |
| Byproducts | Acetyl-CoA, enters TCA cycle | Pyruvate, leads to lactate or acetyl-CoA |
| Regulation | Inhibited by malonyl-CoA | Controlled by phosphofructokinase |
| Preferred During | Fasting, endurance exercise | High-intensity sprints, fed state |
These pathways interconnect; excess acetyl-CoA from beta oxidation can inhibit glycolysis via the Randle cycle, prioritizing fat use. In cancer cells, glycolysis often dominates (Warburg effect), while healthy cells balance both for optimal efficiency.
FAQ 14: What Are the Evolutionary Aspects of Beta Oxidation?
Beta oxidation’s origins trace back to ancient prokaryotes, where it occurred in the cytosol for basic energy needs from lipids. As eukaryotes evolved, the pathway shifted to mitochondria, likely from bacterial endosymbionts, coupling it tightly with oxidative phosphorylation for higher ATP yields. This adaptation allowed complex organisms to store and utilize fats efficiently, crucial for survival in fluctuating environments.
In mammals, peroxisomal variants emerged for handling very long-chain fats, adding redundancy and specialization. Evolutionary pressures favored enzymes with broad substrate specificity, enabling diverse diets. For instance, in hibernating animals, upregulated beta oxidation genes support prolonged fat reliance, mirroring human adaptations during starvation.
This conservation across species underscores its role in energy homeostasis, with mutations leading to disorders highlighting selective pressures. Future insights may reveal how climate-driven dietary shifts influenced pathway evolution in early humans.
FAQ 15: Which Supplements Can Enhance Beta Oxidation?
Supplements targeting beta oxidation aim to boost fat metabolism, often appealing to athletes or those seeking weight management. L-carnitine stands out, facilitating fatty acid transport into mitochondria via the carnitine shuttle, potentially increasing oxidation rates during exercise. Studies show it elevates palmitate oxidation in cells, though effects vary by dosage and individual.
Other options include:
- Green Tea Extract: Contains catechins that may raise resting fat oxidation by up to 10 percent, supporting energy expenditure.
- Omega-3 Fatty Acids: From fish oil, they enhance mitochondrial function and glycolytic metabolism, indirectly aiding beta oxidation.
- Caffeine: Boosts fatty acid mobilization, making more substrate available for the pathway.
- Conjugated Linoleic Acid (CLA): Promotes fat breakdown and may increase enzyme activity in the cycle.
While promising, supplements work best with diet and exercise; over-reliance without evidence can be ineffective. Consult professionals, as high doses might cause side effects like gastrointestinal issues.
FAQ 16: How Does Beta Oxidation Differ in Plants Versus Animals?
In animals, beta oxidation primarily occurs in mitochondria, generating ATP through electron carriers like NADH and FADH2, essential for energy during fasting. Peroxisomes handle very long-chain fats, producing hydrogen peroxide as a byproduct, detoxified by catalase. This dual system allows efficient handling of dietary and stored lipids.
Plants, however, conduct beta oxidation exclusively in peroxisomes and glyoxysomes, linking it to gluconeogenesis rather than direct ATP production. During seed germination, it converts stored oils to sugars for growth, with no mitochondrial involvement. Enzymes differ; plant acyl-CoA oxidases use oxygen directly, yielding H2O2 for signaling or defense.
These variations reflect evolutionary adaptations: animals prioritize energy yield, while plants focus on carbon recycling. Fungi mirror plants, emphasizing peroxisomal activity.
FAQ 17: What Happens to Beta Oxidation During Exercise?
During exercise, beta oxidation ramps up to meet energy demands, especially in endurance activities where fats become a primary fuel after glycogen depletion. Moderate-intensity workouts, like jogging, maximize fat oxidation rates, influenced by training status and duration. Elite athletes show higher capacities due to increased mitochondrial enzymes.
Regulation involves:
- Increased Mobilization: Hormones like adrenaline enhance lipolysis, supplying more fatty acids.
- Enzyme Activation: AMPK boosts CPT-I activity, facilitating transport into mitochondria.
- Intensity Effects: At high intensities, glycolysis dominates, but fats contribute up to 50 percent at moderate levels.
- Training Adaptations: Regular exercise elevates HAD activity, improving efficiency.
Post-exercise, the pathway aids recovery by replenishing stores. Factors like sex and nutrition affect rates, with females often oxidizing more fats.
FAQ 18: How Is Beta Oxidation Altered in Diabetes?
In diabetes, beta oxidation often increases due to high fatty acid availability from insulin resistance, leading to excess acetyl-CoA and ketone production in type 1, risking ketoacidosis. In type 2, incomplete oxidation in muscles contributes to lipid accumulation, worsening insulin sensitivity via ceramide buildup.
This imbalance stems from disrupted regulation; elevated free fatty acids overwhelm mitochondria, causing oxidative stress. Hearts in diabetics rely more on fats, reducing efficiency and promoting cardiomyopathy. Therapies aim to restore balance, like metformin enhancing glucose use to curb fat oxidation.
Paradoxically, some studies show decreased capacity in advanced stages, highlighting the need for targeted interventions to prevent complications.
FAQ 19: What Foods Can Promote Beta Oxidation?
Foods that promote beta oxidation supply substrates or compounds enhancing the pathway, aiding fat metabolism for energy or weight control. Fatty fish like salmon provide omega-3s, boosting mitochondrial function and oxidation rates. Avocados offer monounsaturated fats, easily processed without overwhelming the system.
| Food Category | Examples | How They Promote Beta Oxidation |
|---|---|---|
| Healthy Fats | Olive oil, nuts | Supply medium-chain triglycerides for quick entry |
| Proteins | Lean meats, eggs | Support enzyme synthesis; high-protein diets may enhance rates |
| Vegetables | Broccoli, spinach | Rich in biotin for related carboxylases |
| Fruits | Berries, citrus | Antioxidants reduce oxidative stress on mitochondria |
| Whole Grains | Oats, quinoa | Provide fiber to stabilize blood sugar, favoring fat use |
| Beverages | Green tea | Catechins increase resting oxidation |
Avoid high-sugar foods that inhibit via insulin spikes. Combined with exercise, these choices optimize the process.
FAQ 20: What Does Future Research Hold for Beta Oxidation?
Future research on beta oxidation promises advances in treating metabolic diseases, focusing on its role in cancer, immunity, and neurodegeneration. Scientists are exploring how modulating the pathway could combat drug resistance in tumors, where upregulated oxidation supports survival. Gene therapies for oxidation disorders aim to correct enzyme deficiencies, improving energy production.
In aging and Alzheimer’s, enhancing neuronal fat use might preserve cognitive function, as recent studies link it to memory consolidation. Climate impacts on diets could drive evolutionary studies, while AI models predict pathway overloads for personalized nutrition.
Overall, integrating omics data will uncover novel regulators, paving the way for drugs that fine-tune oxidation without side effects.
Acknowledgement
The Examsmeta.com website expresses its gratitude to several reputable sources that provided valuable insights and detailed information for the article “Beta Oxidation: Fatty Acid Metabolism, Energy Production, and Health.” Their comprehensive resources on biochemistry, metabolism, and related clinical aspects greatly enriched the content, ensuring accuracy and depth. Specifically, acknowledges the contributions of:
- PubMed (pubmed.ncbi.nlm.nih.gov) for its extensive database of peer-reviewed scientific studies on beta oxidation and fatty acid metabolism.
- ScienceDirect (www.sciencedirect.com) for offering in-depth articles on enzymatic processes and metabolic regulation.
- National Center for Biotechnology Information (NCBI) (www.ncbi.nlm.nih.gov) for its detailed biochemical pathways and genetic disorder references.
- Khan Academy (www.khanacademy.org) for its clear explanations of metabolic processes, making complex concepts accessible.
- Nature (www.nature.com) for cutting-edge research on evolutionary and clinical aspects of fatty acid oxidation.

