The microscopic world of cells is a fascinating realm where life’s smallest units perform extraordinary tasks to sustain living organisms. At the heart of this cellular universe lies the cell envelope, a critical structure in prokaryotic cells, such as bacteria, that serves as a protective barrier and functional hub.
This article delves into the definition, classification, types, and functions of the cell envelope, weaving together detailed explanations, real-world examples, and insights into its vital role in bacterial survival. Whether you’re a biology enthusiast or simply curious about the microscopic machinery of life, this exploration will illuminate the wonders of the cell envelope in simple, engaging language.
Table of Contents
What Is the Cell Envelope?
The cell envelope is the outermost protective covering of prokaryotic cells, acting as a fortress that safeguards the cell’s internal environment. It is a complex, multi-layered structure that typically includes the plasma membrane, cell wall, and, in some cases, an outer membrane or additional layers like the glycocalyx. Think of it as a multi-tiered defense system, not unlike a medieval castle with walls, gates, and moats, each layer contributing to the cell’s structural integrity and functionality. This envelope not only shields the cell from external threats but also regulates the flow of nutrients and waste, ensuring the cell thrives in diverse environments.
In prokaryotes, such as bacteria, the cell envelope is essential for maintaining the cell’s shape and protecting it from bursting under internal pressure. It also plays a pivotal role in processes like nutrient uptake, protein secretion, and even communication with the external world. For instance, a bacterium navigating a harsh environment, like a hot spring or a human gut, relies on its cell envelope to withstand extreme conditions while facilitating essential functions.
Classification of the Cell Envelope
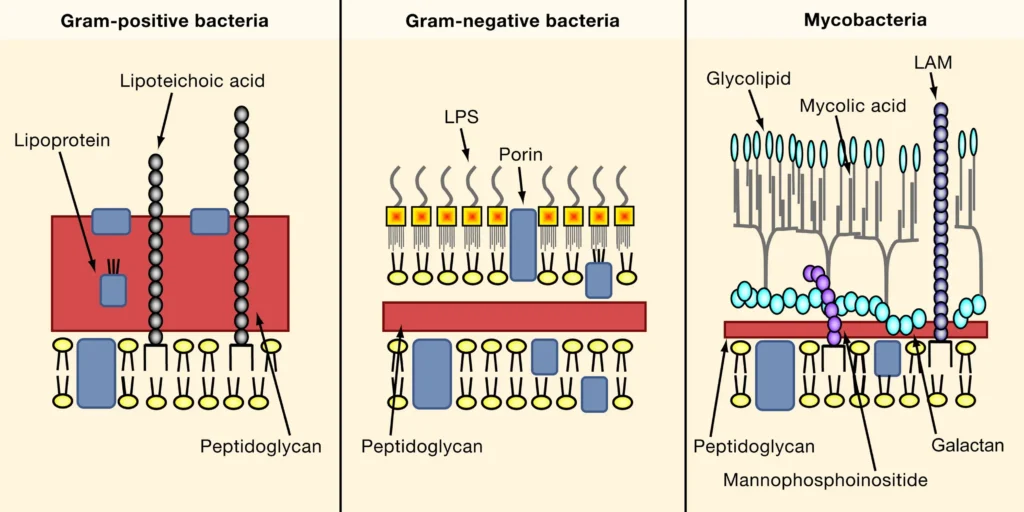
Bacteria are incredibly diverse, and their cell envelopes reflect this variety. Based on their response to the Gram staining technique—a method used to differentiate bacteria by staining them with a purple dye—bacteria are classified into three main groups: Gram-positive, Gram-negative, and Mycobacteria. Each group has a distinct cell envelope structure, which influences its behavior, survival strategies, and interactions with the environment.
Gram-Positive Bacteria
Gram-positive bacteria boast a thick peptidoglycan layer in their cell wall, which acts like a sturdy brick wall, providing rigidity and strength. This layer traps the purple dye during Gram staining, giving these bacteria a vibrant violet appearance under a microscope. The peptidoglycan is interwoven with teichoic acids and lipoteichoic acids, which contribute to the cell’s negative charge and help anchor the cell wall to the plasma membrane. These acids also play a role in cell division and interactions with the host environment.
For example, Staphylococcus aureus, a common Gram-positive bacterium, relies on its robust cell envelope to survive on human skin and resist certain antibiotics. The thick peptidoglycan layer makes it challenging for some drugs to penetrate, highlighting the envelope’s protective prowess.
Gram-Negative Bacteria
In contrast, Gram-negative bacteria have a thinner peptidoglycan layer, sandwiched between an inner plasma membrane and an outer lipid bilayer containing lipopolysaccharides (LPS). This outer membrane acts as an additional shield, making Gram-negative bacteria more resistant to certain antibiotics and environmental stresses. However, the thin peptidoglycan layer fails to retain the purple dye during Gram staining, causing these bacteria to appear pink or red after counterstaining.
Escherichia coli, a well-known Gram-negative bacterium found in the human gut, exemplifies this structure. Its outer membrane, rich in LPS, protects it from bile salts in the digestive tract while allowing selective nutrient uptake. The periplasmic space—a compartment between the plasma membrane and outer membrane—houses enzymes that aid in nutrient processing and detoxification, showcasing the cell envelope’s multifunctional nature.
Mycobacteria: A Unique Case
Mycobacteria stand out with a cell envelope that deviates from the Gram-positive and Gram-negative blueprints. Instead of an outer membrane, they possess a complex cell wall rich in mycolic acids, arabinogalactan, and peptidoglycan. This lipid-heavy wall, which can constitute up to 60% of the cell’s dry weight, forms a waxy, impermeable barrier. This unique structure makes mycobacteria, like Mycobacterium tuberculosis (the causative agent of tuberculosis), notoriously resistant to antibiotics and immune responses.
The mycolic acid layer is like a suit of armor, shielding the bacterium from harsh conditions, such as the acidic environment of a macrophage (an immune cell). This resilience explains why treating tuberculosis requires prolonged antibiotic regimens, as the cell envelope poses a formidable barrier to drug penetration.
Other Variations: Mollicutes and Chlamydiaceae
Some bacteria defy conventional cell envelope structures. Mollicutes, such as Mycoplasma, lack a cell wall entirely, relying solely on a plasma membrane reinforced with sterols for stability. This absence makes them highly flexible but also vulnerable to environmental stresses, which is why they often thrive as intracellular parasites. For example, Mycoplasma pneumoniae causes walking pneumonia in humans, exploiting its host’s resources while evading external threats.
Similarly, Chlamydiaceae, like Chlamydia trachomatis, have an unusual cell envelope. Their infectious forms lack detectable peptidoglycan, instead relying on a layer of disulfide bond cross-linked proteins to maintain structural integrity. This adaptation allows Chlamydia to survive within host cells, contributing to its role as a leading cause of bacterial sexually transmitted infections.
| Bacterial Group | Cell Envelope Features | Examples | Gram Staining Result |
|---|---|---|---|
| Gram-Positive | Thick peptidoglycan layer, teichoic and lipoteichoic acids, no outer membrane | Staphylococcus aureus | Purple (retains dye) |
| Gram-Negative | Thin peptidoglycan layer, outer membrane with LPS, periplasmic space | Escherichia coli | Pink (counterstained) |
| Mycobacteria | Lipid-rich cell wall with mycolic acids, arabinogalactan, no outer membrane | Mycobacterium tuberculosis | Not clearly Gram-positive or negative |
| Mollicutes | No cell wall, only plasma membrane with sterols | Mycoplasma pneumoniae | Not applicable |
| Chlamydiaceae | No detectable peptidoglycan in infectious forms, disulfide-linked protein layer | Chlamydia trachomatis | Gram-negative-like |
Components of the Cell Envelope
The cell envelope is a sophisticated assembly of layers, each with distinct compositions and roles. These layers work in harmony to protect the cell, regulate its interactions, and maintain its structural integrity. Let’s break down the key components.
Glycocalyx: The Outer Shield
The glycocalyx is the outermost layer of the cell envelope in many bacteria, resembling a sticky, sugary coating. Composed of polysaccharides and sometimes proteins, it varies in thickness and structure, appearing as either a loose slime layer or a dense capsule. The slime layer, found in bacteria like Pseudomonas aeruginosa, protects against dehydration and antibiotics, enabling the bacterium to form biofilms on medical devices. The capsule, seen in Streptococcus pneumoniae, is thicker and tougher, preventing phagocytosis—the process by which immune cells engulf pathogens.
The glycocalyx also aids in adhesion, allowing bacteria to stick to surfaces or host tissues. For instance, Streptococcus mutans uses its glycocalyx to adhere to tooth enamel, contributing to dental plaque formation. This layer’s versatility makes it a critical survival tool in diverse environments.
Cell Wall: The Structural Backbone
The cell wall provides the bacterium with its shape and rigidity, preventing it from bursting under internal hydrostatic pressure. In most bacteria, the cell wall is composed of peptidoglycan, a polymer of sugars and amino acids that forms a mesh-like structure. In Gram-positive bacteria, this layer is thick and robust, while in Gram-negative bacteria, it is thinner but supported by the outer membrane.
In mycobacteria, the cell wall’s high lipid content, driven by mycolic acids, creates an exceptionally tough barrier. This lipid-rich wall not only protects against antibiotics but also contributes to the bacterium’s ability to persist in hostile environments, such as within granulomas in tuberculosis patients.
Plasma Membrane: The Gatekeeper
The plasma membrane, a lipid bilayer embedded with proteins, serves as the cell’s primary interface with the outside world. It is semi-permeable, selectively allowing nutrients in and waste out while maintaining the cell’s internal environment. The plasma membrane is also a hub for critical processes like energy production, protein secretion, and chromosome segregation.
In some bacteria, the plasma membrane forms specialized structures called mesosomes, which are invaginations that increase surface area for functions like respiration and cell wall synthesis. In cyanobacteria, chromatophores—membrane extensions containing pigments—enable photosynthesis, illustrating the plasma membrane’s adaptability.
| Component | Composition | Function |
|---|---|---|
| Glycocalyx | Polysaccharides, proteins | Protection, adhesion, prevents phagocytosis (capsule), biofilm formation |
| Cell Wall | Peptidoglycan, mycolic acids (in mycobacteria) | Structural support, shape maintenance, withstands hydrostatic pressure |
| Plasma Membrane | Lipid bilayer, proteins | Nutrient transport, energy production, protein secretion, environmental sensing |
| Outer Membrane | Lipopolysaccharides, phospholipids (Gram-negative only) | Barrier to antibiotics, selective permeability, protection from host defenses |
Functions of the Cell Envelope
The cell envelope is far more than a passive barrier; it is a dynamic structure that orchestrates a range of essential functions. These functions ensure bacterial survival, adaptability, and interaction with their surroundings.
Structural Integrity and Shape Maintenance
The cell envelope, particularly the cell wall, provides structural integrity, preventing the cell from bursting due to internal osmotic pressure. Prokaryotes often live in hypotonic environments, where water tends to flow into the cell. The rigid cell wall counteracts this pressure, maintaining the cell’s shape—whether rod-shaped (Bacillus), spherical (Staphylococcus), or spiral (Spirillum). Without this support, the cell would swell and rupture, much like an overinflated balloon.
Protection Against Environmental Stresses
The cell envelope acts as a shield against external threats, such as antibiotics, immune responses, and extreme conditions. The glycocalyx protects against dehydration and phagocytosis, while the outer membrane in Gram-negative bacteria blocks many antibiotics. Mycobacteria’s mycolic acid layer offers unparalleled resistance, enabling survival in harsh settings like acidic or oxygen-poor environments.
For example, Pseudomonas aeruginosa uses its glycocalyx to form biofilms, which protect it from antibiotics and immune attacks in chronic infections, such as those in cystic fibrosis patients. This protective role underscores the cell envelope’s importance in bacterial persistence.
Regulation of Nutrient and Waste Transport
The plasma membrane and periplasmic space (in Gram-negative bacteria) regulate the flow of molecules into and out of the cell. The plasma membrane’s selective permeability ensures that essential nutrients, like sugars and ions, enter while toxic substances are excluded. The periplasmic space contains enzymes that break down complex molecules, facilitating nutrient uptake.
For instance, Escherichia coli uses its periplasmic enzymes to degrade sugars, which are then transported across the plasma membrane for energy production. This coordinated transport system is vital for bacterial metabolism and growth.
Facilitation of Cellular Processes
The cell envelope is a hub for critical processes like protein secretion, cell division, and energy production. The plasma membrane houses enzymes involved in respiration and ATP synthesis, while mesosomes in some bacteria enhance these processes by increasing membrane surface area. In Gram-negative bacteria, the outer membrane facilitates the secretion of virulence factors, enabling pathogens like Salmonella to infect host cells.
Additionally, the cell envelope plays a role in chromosomal segregation during cell division, ensuring genetic material is properly distributed. In cyanobacteria, chromatophores enable photosynthesis, highlighting the envelope’s role in specialized functions.
Interaction with the Host and Environment
The cell envelope mediates interactions with the environment, including adhesion to surfaces and evasion of host defenses. The glycocalyx enables bacteria to stick to tissues or medical devices, forming biofilms that complicate treatment. For example, Streptococcus pneumoniae’s capsule prevents phagocytosis, allowing it to cause pneumonia.
In Gram-negative bacteria, lipopolysaccharides trigger immune responses in hosts, which can be both a defense mechanism and a contributor to disease severity, as seen in sepsis caused by E. coli. These interactions highlight the cell envelope’s role in bacterial pathogenesis and survival.
The Cell Envelope in Action
The cell envelope’s structure and functions have profound implications in medicine, biotechnology, and environmental science. Understanding its role helps scientists develop antibiotics, combat infections, and harness bacteria for industrial purposes.
Antibiotic Resistance and Drug Development
The cell envelope is a primary target for antibiotics. Drugs like penicillin inhibit peptidoglycan synthesis, weakening the cell wall and causing bacterial lysis. However, the diverse envelope structures—particularly the outer membrane in Gram-negative bacteria and the mycolic acid layer in mycobacteria—pose challenges to antibiotic efficacy. For instance, Mycobacterium tuberculosis’s lipid-rich wall requires specialized drugs like isoniazid, which target mycolic acid synthesis.
Research into novel antibiotics often focuses on disrupting cell envelope components, such as LPS or teichoic acids, to overcome resistance. Understanding these structures also aids in developing vaccines, as components like LPS and capsules are often immunogenic.
Biofilm Formation and Medical Challenges
Biofilms, driven by the glycocalyx, are a major challenge in healthcare. Bacteria like Pseudomonas aeruginosa form biofilms on catheters and implants, resisting antibiotics and immune responses. The glycocalyx’s protective properties make these infections difficult to treat, necessitating innovative strategies like quorum-sensing inhibitors to disrupt biofilm formation.
Environmental and Industrial Applications
In environmental contexts, the cell envelope enables bacteria to thrive in extreme conditions, such as acidic mine drainage or deep-sea vents. Biotechnologists exploit these properties for bioremediation, using bacteria like Pseudomonas to degrade pollutants. The cell envelope’s ability to regulate nutrient uptake and withstand stress makes these bacteria ideal for such applications.
In industry, bacteria with specialized cell envelopes, like cyanobacteria with chromatophores, are used for biofuel production. Their photosynthetic capabilities, driven by the cell envelope, offer sustainable energy solutions.
Conclusion
The cell envelope is a remarkable structure, blending strength, flexibility, and functionality to ensure bacterial survival. From the sturdy peptidoglycan walls of Gram-positive bacteria to the lipid-rich barriers of mycobacteria, each variation reflects an evolutionary adaptation to specific challenges. The envelope’s roles—protecting against threats, regulating transport, and facilitating critical processes—underscore its importance as a cornerstone of prokaryotic life.
By studying the cell envelope, scientists unlock insights into bacterial behavior, paving the way for advancements in medicine, biotechnology, and environmental science. Whether it’s combating antibiotic resistance, harnessing bacteria for industry, or marveling at their resilience, the cell envelope remains a testament to the ingenuity of life at the microscopic level. Next time you think of bacteria, picture the cell envelope as their unsung hero—a dynamic shield that enables these tiny organisms to conquer the toughest environments.
Read These Articles in Detail
- The Cell: The Fundamental Building Block of Life
- Prokaryotic Cells vs. Eukaryotic Cells: A Detailed Exploration
- Prokaryotic Cells: Definition, Anatomy, Functions, and Their Importance
- Eukaryotic Cells Explained: Definition, Structure, Functions, and Importance
- The Cell Membrane: Structure, Functions, and Evolutionary Significance
- The Cell Wall: Structure, Composition, Functions, and Evolutionary Significance
- Endomembrane System: A Detailed Exploration of the Cellular Machinery of Life
- Mitochondria: A Comprehensive Guide to Their Structure and Functions
- The Cell Envelope: The Protective Shield of Prokaryotic Cells
- Ribosomes: Definition, Structure, and Functions
- Endoplasmic Reticulum: Structure, Types, and Functions Explained
- The Golgi Apparatus: Structure, Functions, and Cellular Importance
- Cytoplasm and Nucleus: Structure, Functions, Differences, and Examples
- What Is Cell Signaling? Structure, Types, and Functions Explained
- Three Main Parts of a Nucleotide: Structure and Functions
- Enzymes: Definition, Structure, Classification, and Functions
- DNA: Structure, Function, Discovery, and Modern Applications
- DNA Replication: Steps, Process, and Genetic Importance
- Properties of DNA: Structure, Physical, and Chemical Characteristics
- DNA Packaging: How DNA Fits into the Nucleus of a Cell
- R-Factors and Plasmids: How Bacteria Develop Antibiotic Resistance
- Chromosomes: Structure, Types, Functions, and Role in Genetics
- Genetic Material: DNA, RNA, and Their Role in Heredity and Evolution
- Deoxyribose and Ribose: Structure and Function in DNA and RNA
- Differences Between Genes and DNA: Definitions, Structures, and Functions
- RNA: Definition, Structure, Types, Functions, and Its Role in Life
Frequently Asked Questions
FAQ 1: What is the cell envelope in bacteria, and why is it important?
The cell envelope is the protective outer covering of prokaryotic cells, like bacteria, that acts as a shield and functional hub. It typically includes the plasma membrane, cell wall, and, in some cases, an outer membrane or glycocalyx. Think of it as a fortress that keeps the cell safe while allowing it to interact with its environment. This structure is crucial for maintaining the cell’s shape, protecting it from external threats, and regulating the flow of nutrients and waste.
The importance of the cell envelope lies in its multifaceted roles. It provides structural integrity, preventing the cell from bursting under internal pressure, especially in environments where water flows into the cell. It also acts as a barrier against antibiotics, immune responses, and harsh conditions like extreme pH or temperature. For example, bacteria like Escherichia coli rely on their cell envelope to survive in the human gut, where they face bile salts and immune cells. Additionally, the envelope facilitates essential processes like protein secretion and energy production, making it a cornerstone of bacterial survival and adaptability.
FAQ 2: How does the cell envelope differ between Gram-positive and Gram-negative bacteria?
The cell envelope of Gram-positive and Gram-negative bacteria differs significantly, primarily due to their cell wall composition and structure, which affects how they appear under Gram staining. Gram-positive bacteria have a thick peptidoglycan layer in their cell wall, interwoven with teichoic acids and lipoteichoic acids. This robust layer traps the purple dye during Gram staining, giving these bacteria a violet color. The thick wall provides strong structural support and makes them less permeable to certain antibiotics.
In contrast, Gram-negative bacteria have a thinner peptidoglycan layer sandwiched between an inner plasma membrane and an outer lipid bilayer containing lipopolysaccharides (LPS). This outer membrane makes them appear pink or red after Gram staining, as the thin peptidoglycan layer cannot retain the purple dye. The outer membrane acts as an additional barrier, protecting against antibiotics and environmental stresses. For instance, Pseudomonas aeruginosa, a Gram-negative bacterium, uses its outer membrane to resist antibiotics in hospital settings. The periplasmic space between the membranes in Gram-negative bacteria also houses enzymes for nutrient processing, adding another layer of functionality.
FAQ 3: What makes the cell envelope of mycobacteria unique?
Mycobacteria, such as Mycobacterium tuberculosis, have a cell envelope that stands out due to its unique composition and structure. Unlike Gram-positive or Gram-negative bacteria, mycobacteria lack an outer membrane but possess a highly lipid-rich cell wall made of mycolic acids, arabinogalactan, and peptidoglycan. This wall can constitute up to 60% of the cell’s dry weight, making it exceptionally thick and waxy, almost like a suit of armor.
This lipid-heavy structure provides remarkable resistance to antibiotics, immune responses, and harsh environments, such as the acidic interior of immune cells. For example, the mycolic acid layer in Mycobacterium tuberculosis allows it to persist in the human body for years, contributing to the difficulty of treating tuberculosis. The unique envelope also affects Gram staining, as mycobacteria do not clearly fit into the Gram-positive or Gram-negative categories, often requiring special staining techniques like acid-fast staining to identify them.
FAQ 4: What is the role of the glycocalyx in the bacterial cell envelope?
The glycocalyx is a sugary, outermost layer of the cell envelope in many bacteria, composed of polysaccharides and sometimes proteins. It exists in two forms: a loose slime layer or a thick, tough capsule. This layer plays a critical role in protecting bacteria and helping them interact with their environment. For instance, the glycocalyx shields bacteria from dehydration, antibiotics, and immune attacks, making it a key survival tool.
The slime layer, found in bacteria like Pseudomonas aeruginosa, enables biofilm formation, allowing bacteria to stick to surfaces like medical devices or tissues. Biofilms make infections harder to treat, as seen in chronic lung infections in cystic fibrosis patients. The capsule, present in bacteria like Streptococcus pneumoniae, prevents phagocytosis, where immune cells engulf pathogens, thus aiding in disease causation, such as pneumonia. Additionally, the glycocalyx helps bacteria adhere to surfaces, like Streptococcus mutans sticking to teeth to form dental plaque.
FAQ 5: How does the cell wall contribute to bacterial survival?
The cell wall is a critical component of the cell envelope, providing structural integrity and maintaining the cell’s shape. Composed primarily of peptidoglycan in most bacteria, it acts like a rigid scaffold that prevents the cell from bursting under hydrostatic pressure caused by water influx in hypotonic environments. For example, rod-shaped bacteria like Bacillus owe their structure to the cell wall’s strength.
Beyond structural support, the cell wall protects against environmental stresses, such as antibiotics targeting peptidoglycan synthesis (e.g., penicillin). In Gram-positive bacteria, the thick peptidoglycan layer enhances resistance, while in Gram-negative bacteria, the cell wall works with the outer membrane for added protection. In mycobacteria, the lipid-rich cell wall, with mycolic acids, provides exceptional resistance, allowing survival in harsh conditions, like inside immune cells. The cell wall also facilitates processes like cell division and nutrient transport, making it indispensable for bacterial survival.
FAQ 6: What functions does the plasma membrane serve in the cell envelope?
The plasma membrane, a lipid bilayer embedded with proteins, is the innermost layer of the cell envelope and serves as the cell’s primary interface with its environment. It is semi-permeable, controlling the entry of nutrients like sugars and ions while expelling waste. This selective transport ensures the cell maintains an optimal internal environment, crucial for survival.
The plasma membrane is also a hub for vital processes. It houses enzymes for energy production through respiration and ATP synthesis, supports protein secretion, and aids in chromosomal segregation during cell division. In some bacteria, mesosomes—extensions of the plasma membrane—increase surface area for these functions. For example, in cyanobacteria, chromatophores in the plasma membrane contain pigments for photosynthesis, enabling energy production. The membrane’s versatility makes it essential for bacterial metabolism and adaptability.
FAQ 7: How does the cell envelope contribute to antibiotic resistance?
The cell envelope is a key player in antibiotic resistance, acting as a barrier that prevents drugs from reaching their targets. In Gram-positive bacteria, the thick peptidoglycan layer can block some antibiotics, though drugs like penicillin target peptidoglycan synthesis to weaken the cell wall. In Gram-negative bacteria, the outer membrane with lipopolysaccharides (LPS) acts as an additional shield, limiting antibiotic penetration. For instance, Pseudomonas aeruginosa resists many drugs due to its outer membrane.
Mycobacteria, like Mycobacterium tuberculosis, have a lipid-rich cell wall with mycolic acids, making them highly resistant to antibiotics and requiring specialized drugs like isoniazid. The glycocalyx, particularly in biofilm-forming bacteria, further enhances resistance by shielding cells from antibiotics and immune responses. Understanding these mechanisms drives research into new antibiotics that can bypass or disrupt the cell envelope’s defenses.
FAQ 8: What is the periplasmic space, and what is its role in Gram-negative bacteria?
The periplasmic space is a unique compartment in Gram-negative bacteria, located between the plasma membrane and the outer membrane. This space contains a thin peptidoglycan layer and a gel-like matrix filled with enzymes and proteins. It plays a critical role in enhancing the functionality of the cell envelope by supporting processes like nutrient processing and detoxification.
The periplasmic space houses enzymes that break down complex molecules, such as sugars, into forms that can be transported across the plasma membrane. For example, Escherichia coli uses periplasmic enzymes to degrade nutrients in the gut, aiding metabolism. It also contains proteins that detoxify harmful substances, contributing to antibiotic resistance. Additionally, the periplasmic space supports protein folding and transport, ensuring the cell can secrete virulence factors or signaling molecules, as seen in pathogens like Salmonella.
FAQ 9: How do bacteria without a cell wall, like Mollicutes, survive?
Mollicutes, such as Mycoplasma pneumoniae, are unique bacteria that lack a cell wall, relying solely on a reinforced plasma membrane for their cell envelope. The plasma membrane incorporates sterols, which provide stability and flexibility, compensating for the absence of a rigid cell wall. This makes Mollicutes highly adaptable but also vulnerable to environmental stresses like osmotic changes or antibiotics targeting peptidoglycan.
To survive, Mollicutes often live as intracellular parasites, exploiting host resources to avoid external threats. For example, Mycoplasma pneumoniae thrives in the human respiratory tract, causing walking pneumonia. Their flexible shape allows them to evade immune responses and adhere to host cells. This adaptation highlights the cell envelope’s critical role, even in its simplest form, in enabling bacterial survival in specific niches.
FAQ 10: How does the cell envelope aid in bacterial interactions with their environment?
The cell envelope is a dynamic interface that mediates bacterial interactions with their environment, enabling adhesion, immune evasion, and communication. The glycocalyx, for instance, allows bacteria to stick to surfaces, forming biofilms or adhering to host tissues. Streptococcus mutans uses its glycocalyx to attach to teeth, contributing to dental plaque, while Pseudomonas aeruginosa forms biofilms on medical devices, complicating infections.
The outer membrane in Gram-negative bacteria, with lipopolysaccharides (LPS), triggers host immune responses but also protects against immune attacks. In mycobacteria, the mycolic acid layer enables survival within immune cells, as seen in Mycobacterium tuberculosis. The plasma membrane facilitates nutrient uptake and signaling, allowing bacteria to sense and respond to environmental changes. These interactions make the cell envelope a key player in bacterial pathogenesis, environmental adaptation, and biotechnological applications like bioremediation.
FAQ 11: What are the main components of the bacterial cell envelope?
The cell envelope in bacteria is a complex, multi-layered structure that serves as a protective and functional barrier. It typically consists of three primary components: the glycocalyx, cell wall, and plasma membrane, with some bacteria also possessing an outer membrane. Each component plays a distinct role, working together to maintain the cell’s integrity and facilitate interactions with the environment. For example, a bacterium like Escherichia coli relies on its cell envelope to survive the harsh conditions of the human gut.
The glycocalyx, the outermost layer, is a sticky coating of polysaccharides and sometimes proteins, appearing as either a loose slime layer or a thick capsule. It protects against environmental threats like antibiotics and aids in adhesion, as seen in Streptococcus mutans, which forms dental plaque. The cell wall, primarily made of peptidoglycan, provides structural support, maintaining the cell’s shape and preventing it from bursting. The plasma membrane, a lipid bilayer with embedded proteins, regulates nutrient and waste transport while supporting processes like energy production. In Gram-negative bacteria, an outer membrane with lipopolysaccharides (LPS) adds an extra layer of protection, enhancing resistance to antibiotics.
FAQ 12: How does the cell envelope protect bacteria from environmental stresses?
The cell envelope acts as a robust shield, safeguarding bacteria from a variety of environmental stresses, such as antibiotics, immune responses, and extreme conditions like high salinity or acidity. Its multi-layered structure, including the glycocalyx, cell wall, and plasma membrane, works collectively to ensure bacterial survival. For instance, bacteria in extreme environments, like Deinococcus radiodurans in radioactive settings, rely on their cell envelope to withstand harsh conditions.
The glycocalyx protects against dehydration and immune attacks by forming a barrier, as seen in Pseudomonas aeruginosa, which uses its slime layer to create biofilms that resist antibiotics. The cell wall provides structural strength, countering hydrostatic pressure that could cause the cell to burst in hypotonic environments. In Gram-negative bacteria, the outer membrane blocks many antibiotics and toxins, while in mycobacteria, the mycolic acid-rich cell wall offers exceptional resistance to drugs and immune cells. The plasma membrane further contributes by selectively filtering harmful substances, ensuring the cell’s internal environment remains stable.
FAQ 13: Why is the Gram staining technique important for studying the cell envelope?
Gram staining is a critical laboratory technique used to differentiate bacteria based on their cell envelope structure, specifically the composition of their cell wall. By staining bacteria with a purple dye (crystal violet) and observing whether it is retained or washed away, scientists can classify bacteria as Gram-positive or Gram-negative, which provides insights into their cell envelope properties and behavior. This distinction is vital for understanding bacterial physiology and guiding medical treatments.
Gram-positive bacteria, like Staphylococcus aureus, have a thick peptidoglycan layer that retains the purple dye, appearing violet under a microscope. This thick wall makes them more susceptible to certain antibiotics, like penicillin, which target peptidoglycan synthesis. Gram-negative bacteria, such as Escherichia coli, have a thinner peptidoglycan layer and an outer membrane, causing them to lose the purple dye and appear pink after counterstaining. The outer membrane’s lipopolysaccharides (LPS) contribute to antibiotic resistance, making Gram-negative infections harder to treat. By revealing these structural differences, Gram staining helps researchers identify bacteria and develop targeted therapies.
FAQ 14: How does the cell envelope contribute to bacterial pathogenesis?
The cell envelope plays a pivotal role in bacterial pathogenesis, enabling bacteria to infect hosts, evade immune responses, and cause disease. Its components, including the glycocalyx, cell wall, and outer membrane (in Gram-negative bacteria), equip pathogens with tools to adhere to host tissues, resist immune defenses, and secrete harmful substances. For example, Streptococcus pneumoniae uses its cell envelope to cause pneumonia by evading immune clearance.
The glycocalyx, particularly the capsule, prevents phagocytosis, where immune cells engulf bacteria, as seen in Klebsiella pneumoniae, which causes severe lung infections. In Gram-negative bacteria, lipopolysaccharides (LPS) in the outer membrane trigger strong immune responses, sometimes leading to harmful inflammation, like in sepsis caused by Escherichia coli. The cell wall and plasma membrane also facilitate the secretion of toxins and virulence factors, enabling pathogens like Salmonella to invade host cells. These mechanisms make the cell envelope a key factor in bacterial infectivity and a target for developing vaccines and treatments.
FAQ 15: What is the role of mesosomes in the bacterial cell envelope?
Mesosomes are specialized, invaginated structures formed by the plasma membrane in some bacteria, particularly Gram-positive ones, and are part of the cell envelope. They appear as folded extensions, resembling vesicles, tubules, or lamellae, and increase the membrane’s surface area to support various cellular functions. Though their exact role is debated, mesosomes are believed to enhance processes critical to bacterial survival.
Mesosomes are involved in cell wall synthesis, aiding in the assembly of peptidoglycan during cell division, ensuring the cell maintains its shape. They also play a role in respiration and energy production by housing enzymes for ATP synthesis, similar to mitochondria in eukaryotic cells. Additionally, mesosomes facilitate protein secretion and DNA segregation, ensuring genetic material is properly distributed during cell division. For example, in Bacillus subtilis, mesosomes support efficient cell division in nutrient-rich environments, highlighting their importance in the cell envelope’s functionality.
FAQ 16: How does the cell envelope enable biofilm formation?
Biofilms are communities of bacteria encased in a protective matrix, often causing persistent infections, and the cell envelope, particularly the glycocalyx, is central to their formation. The glycocalyx, composed of polysaccharides and proteins, allows bacteria to adhere to surfaces and each other, forming a sticky, protective layer. This ability is critical in medical and environmental contexts, as biofilms can form on devices like catheters or natural surfaces like rocks in streams.
The slime layer of the glycocalyx, found in bacteria like Pseudomonas aeruginosa, enables adhesion to surfaces and protects against antibiotics and immune responses. In chronic infections, such as those in cystic fibrosis patients, biofilms shield bacteria, making treatment challenging. The cell wall and plasma membrane also contribute by supporting the secretion of matrix components and signaling molecules that coordinate biofilm development. For instance, Staphylococcus epidermidis forms biofilms on medical implants, relying on its cell envelope to maintain structural integrity and resist external threats.
FAQ 17: How does the cell envelope support nutrient uptake in bacteria?
The cell envelope is essential for nutrient uptake, regulating the flow of essential molecules like sugars, amino acids, and ions into the cell while expelling waste. The plasma membrane, with its semi-permeable nature, acts as a gatekeeper, using transport proteins to selectively move nutrients across the membrane. In Gram-negative bacteria, the periplasmic space enhances this process by housing enzymes that break down complex molecules.
For example, Escherichia coli in the human gut uses periplasmic enzymes to degrade sugars, which are then transported across the plasma membrane for energy production. The outer membrane in Gram-negative bacteria contains porins, protein channels that allow small molecules to enter the periplasmic space. In Gram-positive bacteria, the thick peptidoglycan layer is permeable, allowing nutrients to reach the plasma membrane. This coordinated system ensures bacteria efficiently acquire nutrients, supporting growth and survival in diverse environments, from soil to host tissues.
FAQ 18: Why are mycobacteria resistant to many antibiotics?
Mycobacteria, such as Mycobacterium tuberculosis, are notoriously resistant to antibiotics due to their unique cell envelope, particularly their lipid-rich cell wall. This wall contains mycolic acids, arabinogalactan, and peptidoglycan, forming a thick, waxy barrier that constitutes up to 60% of the cell’s dry weight. This structure makes it difficult for antibiotics to penetrate, contributing to the challenge of treating diseases like tuberculosis.
The mycolic acid layer acts like a fortress, blocking many drugs and immune molecules, allowing mycobacteria to survive in harsh environments, such as within macrophages (immune cells). Additionally, the cell envelope’s low permeability limits the uptake of antibiotics, requiring specialized drugs like isoniazid, which target mycolic acid synthesis. The glycocalyx and plasma membrane further enhance resistance by regulating molecule transport and protecting against environmental stresses, making mycobacteria a formidable challenge in medical treatment.
FAQ 19: How does the cell envelope of Chlamydiaceae differ from other bacteria?
Chlamydiaceae, such as Chlamydia trachomatis, have a unique cell envelope that sets them apart from typical Gram-positive or Gram-negative bacteria. Their infectious forms, known as elementary bodies, lack detectable peptidoglycan in their cell wall, instead relying on a layer of disulfide bond-cross-linked proteins between the plasma membrane and outer membrane. This structure provides structural integrity while maintaining flexibility, crucial for their intracellular lifestyle.
This protein-based layer mimics the role of peptidoglycan in other bacteria, but its disulfide bonds are absent in the bacterium’s intracellular forms, making them more fragile. This adaptation allows Chlamydia to survive within host cells, causing infections like sexually transmitted diseases. Unlike mycobacteria or Mollicutes, Chlamydiaceae’s envelope enables them to alternate between infectious and replicative forms, highlighting their specialized strategy for pathogenesis and survival in host environments.
FAQ 20: What are the biotechnological applications of the bacterial cell envelope?
The cell envelope’s unique properties make it a valuable target for biotechnological applications, including bioremediation, drug development, and biofuel production. Its ability to withstand extreme conditions and regulate interactions with the environment allows bacteria to be harnessed for industrial and environmental purposes. For example, bacteria with robust cell envelopes are used to clean up pollutants in contaminated soils and water.
In bioremediation, bacteria like Pseudomonas use their glycocalyx to form biofilms that degrade pollutants, such as oil spills, with the plasma membrane facilitating nutrient uptake for metabolism. In drug development, the cell envelope is a target for new antibiotics, with researchers designing drugs to disrupt peptidoglycan or mycolic acid synthesis. Additionally, cyanobacteria with chromatophores in their cell envelope are used for biofuel production, leveraging their photosynthetic capabilities. The cell envelope’s versatility makes it a cornerstone of biotechnological innovation, addressing environmental and health challenges.
Acknowledgement
The creation of the article “Cell Envelope: The Protective Shield of Prokaryotic Cells” was made possible through the comprehensive insights and detailed information provided by reputable sources. The Examsmeta.com website deeply expresses its gratitude to Nature for its authoritative resources on bacterial cell structures and microbiology, which enriched the article’s depth and accuracy. Additionally, ScienceDirect offered valuable scientific literature that enhanced the understanding of cell envelope functions and classifications. Their contributions were instrumental in crafting a well-rounded and informative piece.
Key points of appreciation:
- Nature provided peer-reviewed insights into bacterial cell envelope diversity and its role in pathogenesis.
- ScienceDirect offered detailed studies on the biochemical composition and biotechnological applications of the cell envelope.

