Contact-dependent signaling is a fascinating aspect of how cells in multicellular organisms stay connected and coordinate their activities. Imagine cells as neighbors in a bustling community; sometimes, they need to chat face-to-face rather than shouting across the street. This type of signaling happens when cells are physically touching, allowing them to exchange information directly through specialized structures.
In animals, this is often managed by channels known as gap junctions, while in plants, similar roles are played by plasmodesmata. These mechanisms ensure that everything from heartbeats to plant growth runs smoothly. By exploring these processes, we can appreciate the intricate ways life maintains harmony at the cellular level.
Table of Contents
This article dives deep into the world of contact-dependent signaling, drawing from biological principles and real-world examples. We’ll cover the basics, delve into structures and functions, highlight practical instances, and even touch on what happens when things go wrong. Whether you’re a student, a science enthusiast, or just curious about how your body works, this comprehensive guide aims to make complex ideas accessible and engaging.
The Essentials of Contact-Dependent Signaling
Contact-dependent signaling requires cells to be in direct physical contact to communicate. This isn’t like sending a text message; it’s more like holding hands and passing notes through your palms. Cells achieve this through receptor-ligand interactions on their surfaces or via tiny channels that link their interiors. These channels allow small molecules, ions, and sometimes even larger substances to flow between cells, enabling coordinated responses.
In multicellular organisms, this signaling is crucial for synchronization. For instance, in tissues where timing is everything, like muscle contractions or developmental processes, direct contact ensures signals aren’t lost in translation. Unlike other forms of cell signaling, such as paracrine or endocrine, which rely on diffusible molecules traveling through extracellular spaces, contact-dependent methods keep things local and precise. This specificity helps prevent miscommunication that could lead to chaos in the organism.
One key advantage of this system is its efficiency in resource use. Cells don’t waste energy broadcasting signals to everyone; they target only their immediate neighbors. This is especially important in dense tissues where rapid, synchronized actions are needed. Researchers have noted that disruptions in these pathways can lead to various disorders, underscoring their importance in health and disease. Overall, contact-dependent signaling forms the backbone of cellular teamwork, allowing organisms to function as unified wholes rather than collections of independent parts.
To break it down further, let’s consider the two main players: gap junctions in animals and plasmodesmata in plants. Each has evolved to suit the unique needs of their respective kingdoms, yet they share the common goal of facilitating intercellular exchange.
Gap Junctions: The Communication Highways in Animal Cells
Gap junctions are specialized structures in animal cells that create direct channels between adjacent cells, allowing for the swift passage of ions, small molecules, and signaling compounds. Picture them as tunnels connecting two houses, where residents can quickly share essentials without stepping outside. These junctions are essential in tissues requiring synchronized activity, such as the heart or smooth muscles.
Structure of Gap Junctions
The building blocks of gap junctions are proteins called connexins. Six connexins come together to form a half-channel known as a connexon in the plasma membrane of one cell. When a connexon from one cell aligns perfectly with another from a neighboring cell, they dock to create a full channel. This channel spans the narrow gap between the two plasma membranes, hence the name gap junction.
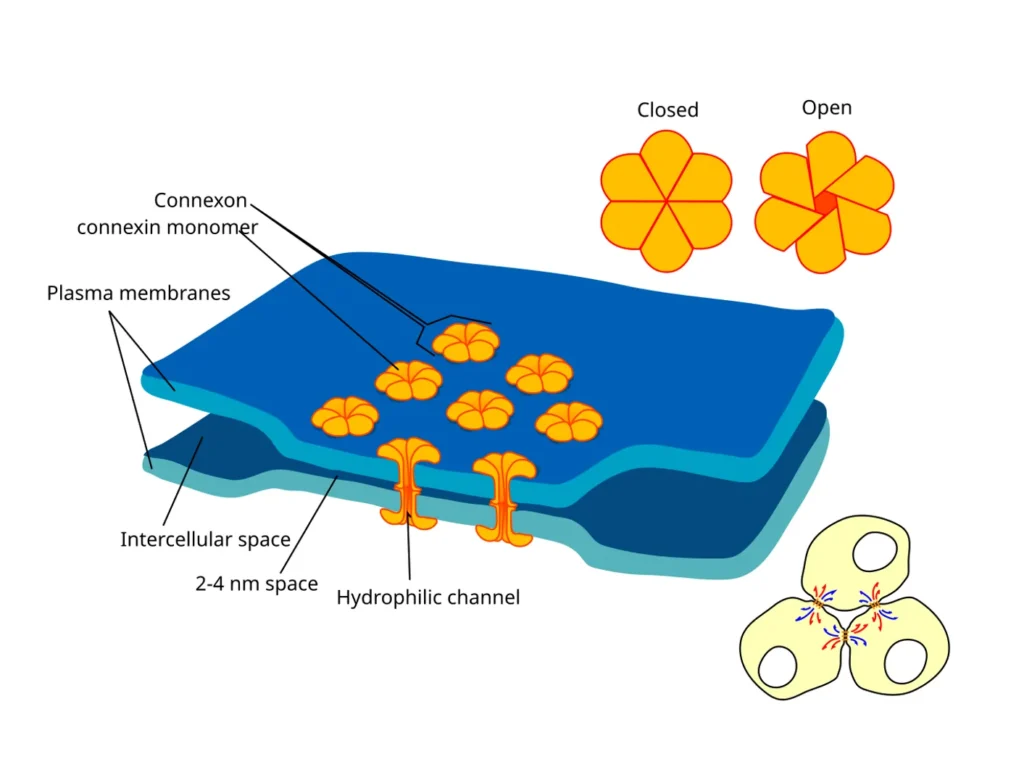
These structures aren’t static; they can open and close in response to cellular conditions. Enzymes and factors like calcium levels or pH changes regulate this gating, ensuring that communication is controlled. In some cases, multiple connexons cluster together to form plaques, amplifying the connection’s capacity. This modular design allows cells to adjust their interconnectivity based on needs, such as during stress or development.
Interestingly, there are different types of connexins, with over 20 identified in humans, each suited to specific tissues. For example, connexin 43 is prevalent in the heart, while others dominate in the nervous system. This diversity adds layers of specificity to how cells communicate.
Functions of Gap Junctions
The primary function of gap junctions is to enable the direct transfer of small molecules and ions between cells. This includes second messengers like cyclic AMP, which help propagate signals rapidly. By allowing electrical and chemical coupling, gap junctions synchronize cellular activities across tissues.
In excitable tissues, this is particularly vital. Electrical impulses can jump from cell to cell through these channels, creating a seamless flow. Beyond that, gap junctions play roles in nutrient sharing, waste removal, and even cell death signaling, where damaged cells can alert neighbors to potential threats.
Regulation is key here. Cells can modulate the permeability of these junctions, deciding what passes through and when. This selectivity helps maintain homeostasis and respond to environmental changes. For instance, during wound healing, gap junctions might close to isolate injured areas, preventing the spread of damage.
Real-World Examples of Gap Junctions in Action
Let’s look at some practical examples to see gap junctions at work. In the heart, these junctions coordinate the rhythmic contraction of cardiac muscle cells. Ions flow through them, ensuring that the electrical signal spreads evenly, leading to a unified heartbeat. Without this, arrhythmias could occur, disrupting the heart’s pumping action.
Another example is in the lens of the eye, where gap junctions help maintain transparency by sharing nutrients and metabolites among cells that lack blood vessels. In smooth muscle tissues, like those in the uterus during labor, these junctions facilitate synchronized contractions, aiding in childbirth.
In the nervous system, gap junctions enable electrical synapses between neurons, allowing faster signal transmission than chemical synapses. This is seen in escape responses in some animals, where quick reflexes are life-saving. Even in non-excitable cells, such as those in the liver, gap junctions assist in metabolic coordination, ensuring efficient detoxification processes.
- Cardiac Synchronization: Ensures uniform ion flow for consistent heartbeats.
- Uterine Contractions: Coordinates muscle activity during labor.
- Neuronal Coupling: Facilitates rapid electrical signaling in brain circuits.
- Epithelial Nutrient Sharing: Supports cells in avascular tissues like the cornea.
- Immune Response Coordination: Allows white blood cells to share signaling molecules during inflammation.
These examples highlight how gap junctions are integral to everyday physiological functions, from beating hearts to clear vision.
Gap Junctions and Diseases
When gap junctions malfunction, it can lead to serious health issues. Mutations in connexin genes are linked to hereditary diseases. For instance, defects in connexin 26 cause congenital deafness by disrupting ion flow in the inner ear. Similarly, skin disorders like keratitis-ichthyosis-deafness syndrome arise from faulty connexins affecting cell communication in the epidermis.
In cardiovascular diseases, altered gap junction function contributes to arrhythmias. During heart attacks, changes in junction permeability can exacerbate damage by allowing harmful substances to spread. Cancer research also shows that reduced gap junction activity promotes tumor growth, as cells lose the ability to suppress uncontrolled division through contact inhibition.
Neurological disorders aren’t spared either. In epilepsy, abnormal gap junctions in astrocytes can lead to synchronized neuronal firing, triggering seizures. Even in conditions like multiple sclerosis, demyelination affects junction integrity, impairing signal transmission.
Understanding these links opens doors to therapies. Drugs that modulate gap junction activity are being explored for treating arrhythmias or enhancing wound healing. This underscores the junctions’ role not just in normal function but in pathology too.
| Disease | Affected Connexin | Symptoms | Mechanism |
|---|---|---|---|
| Congenital Deafness | Connexin 26 | Hearing loss from birth | Disrupted potassium recycling in the cochlea |
| Charcot-Marie-Tooth Disease | Connexin 32 | Nerve damage leading to muscle weakness | Impaired myelin sheath communication in peripheral nerves |
| Oculodentodigital Dysplasia | Connexin 43 | Abnormalities in eyes, teeth, and fingers | Faulty development due to poor cell signaling in embryonic tissues |
| Cataracts | Connexin 50 | Clouding of the eye lens | Reduced nutrient exchange among lens cells |
| Arrhythmogenic Cardiomyopathy | Connexin 43 | Irregular heart rhythms | Altered electrical coupling in cardiac muscles |
| Skin Disorders (e.g., Erythrokeratodermia Variabilis) | Connexin 31 | Scaly skin patches | Defective keratinocyte communication |
| Epilepsy | Various (e.g., Connexin 36 in neurons) | Seizures | Hypersynchronized neuronal activity |
| Cancer (various types) | Multiple connexins | Tumor progression | Loss of growth-inhibitory signals between cells |
This table illustrates just a fraction of the diseases tied to gap junction dysfunction, showing their widespread impact.
Plasmodesmata: Bridging Plant Cells for Growth and Defense
Shifting to the plant world, plasmodesmata serve as the equivalent of gap junctions, but with adaptations suited to plant life. These are cytoplasmic channels that pierce through both the cell membrane and the rigid cell wall, connecting adjacent plant cells. Think of them as secret passages in a fortress, allowing allies to share resources while keeping invaders out.
Structure of Plasmodesmata
Plasmodesmata are more complex than they might seem. At their center lies a tubule from the endoplasmic reticulum called the desmotubule, which runs continuously between cells. Surrounding this is a sleeve of cytoplasm, bounded by the plasma membrane. This setup creates a conduit for molecular traffic.
Unlike the protein-based gap junctions, plasmodesmata involve callose deposits and various proteins that regulate their aperture. They can dilate or constrict, controlling what passes through. Primary plasmodesmata form during cell division, while secondary ones develop later to connect non-sibling cells. This flexibility is key in plant tissues, where cell walls prevent easy movement.
Proteins like plasmodesmata-located proteins (PDLPs) and receptors anchor and regulate these channels, adding layers of control. The structure allows for both passive diffusion of small molecules and active transport of larger ones, making plasmodesmata versatile communication hubs.
Functions of Plasmodesmata
Plasmodesmata enable the symplastic pathway, where substances move cell-to-cell without crossing membranes repeatedly. This is vital for nutrient distribution, such as sugars from photosynthesis traveling through phloem tissues.
They also facilitate signaling by transporting hormones, proteins, and nucleic acids. This helps coordinate growth, development, and responses to stress. For example, plasmodesmata can close during drought to conserve water or open to share defense signals against pests.
Their ability to transport larger molecules like transcription factors influences cell fate and patterning. This is crucial in meristems, where undifferentiated cells decide their roles based on signals from neighbors. Additionally, plasmodesmata play defensive roles, restricting pathogen spread while allowing beneficial exchanges.
Real-World Examples of Plasmodesmata in Plants
In everyday plant life, plasmodesmata are everywhere. During root development, they help distribute auxins, hormones that guide growth patterns. In leaves, they enable the sharing of photosynthetic products, ensuring energy reaches non-photosynthetic parts.
A striking example is in viral defense: while viruses exploit plasmodesmata to spread, plants can deposit callose to seal them off, containing the infection. In flowering, signals through these channels coordinate bloom timing across the plant.
In symbiotic relationships, like with nitrogen-fixing bacteria in legumes, plasmodesmata facilitate nutrient exchange between root cells and nodules. Even in wound responses, they help redirect resources to healing sites.
- Nutrient Transport in Phloem: Sugars move from leaves to roots via symplastic flow.
- Hormone Signaling in Growth: Auxins travel to influence shoot and root elongation.
- Viral Containment: Callose plugs block pathogen movement.
- Developmental Patterning: Transcription factors guide cell differentiation in embryos.
- Stress Responses: Sharing of antioxidants during oxidative stress.
- Symbiosis Support: Exchange in mycorrhizal associations for phosphorus uptake.
- Flowering Coordination: Florigen proteins signal bloom induction.
These instances show plasmodesmata as dynamic players in plant survival and adaptation.
Plasmodesmata in Development and Disease
In plant development, plasmodesmata are pivotal for spatial organization. They allow the movement of morphogens that dictate tissue patterns, such as in leaf vein formation or stomatal spacing. Disruptions can lead to abnormal growth, like stunted roots or malformed leaves.
On the disease front, many plant pathogens target plasmodesmata. Viruses use movement proteins to widen channels for spread, leading to widespread infections like tobacco mosaic virus. Bacterial pathogens might manipulate them too. Fungal invasions can exploit these paths, but plants counter with immune responses that tighten junctions.
Genetic mutations affecting plasmodesmata proteins can cause developmental defects, mirroring animal disorders. Research into enhancing plasmodesmata closure could lead to virus-resistant crops, a boon for agriculture.
| Aspect | Role in Development | Role in Disease |
|---|---|---|
| Transport of Macromolecules | Guides cell fate in meristems by moving transcription factors | Allows viral RNA and proteins to spread between cells |
| Regulation of Aperture | Controls symplastic domains during organ formation | Callose deposition to isolate infected areas |
| Hormone Distribution | Influences root-shoot balance via auxin flow | Pathogens hijack to disrupt growth patterns |
| Nutrient Sharing | Supports embryonic nourishment | Starves healthy cells if channels are blocked by toxins |
| Immune Signaling | Propagates systemic acquired resistance | Exploited by bacteria for effector delivery |
| Structural Integrity | Maintains tissue cohesion in growing shoots | Weakens plants if mutated, leading to susceptibility |
| Response to Stress | Adapts to environmental cues for survival | Amplifies damage in abiotic stresses like salinity |
This table captures the dual roles of plasmodesmata, emphasizing their significance in health and growth.
Comparing Gap Junctions and Plasmodesmata: Similarities and Differences
While gap junctions and plasmodesmata both facilitate direct cell-to-cell communication, they have evolved differently to meet the demands of animal and plant lifestyles. Both allow the passage of small molecules and ions, ensuring coordination, but their structures and regulations differ markedly.
Animals, lacking cell walls, rely on protein-based gap junctions for quick electrical coupling, ideal for rapid responses like heartbeats. Plants, with rigid walls, use plasmodesmata that traverse these barriers, incorporating endoplasmic reticulum elements for broader transport capabilities.
Similarities include selectivity and regulation: both can open or close based on needs. However, plasmodesmata handle larger molecules more readily, aiding in plant-specific processes like virus spread or macromolecular signaling.
| Feature | Gap Junctions (Animals) | Plasmodesmata (Plants) |
|---|---|---|
| Structure | Connexin proteins forming connexons | Desmotubule from ER, surrounded by cytoplasmic sleeve |
| Location | Plasma membrane junctions | Through cell walls and membranes |
| Permeability | Mainly small ions and metabolites (up to 1 kDa) | Small to large molecules, including proteins and RNA |
| Regulation | Gated by pH, calcium, phosphorylation | Controlled by callose, dilation for larger cargo |
| Primary Function | Electrical and chemical coupling | Symplastic transport and signaling |
| Examples in Use | Heart synchronization, neuronal synapses | Nutrient flow in phloem, developmental patterning |
| Disease Association | Arrhythmias, deafness, cancer | Viral infections, developmental defects |
| Evolutionary Adaptation | Suited for mobile, excitable tissues | Adapted for walled, sessile organisms |
This comparison table highlights how these structures, while analogous, are tailored to their environments, showcasing nature’s ingenuity.
The Broader Importance of Contact-Dependent Signaling in Multicellular Life
Contact-dependent signaling isn’t just a cellular quirk; it’s fundamental to multicellularity. In animals, it enables complex behaviors, from immune responses where cells touch to exchange alerts, to embryonic development where contact guides tissue formation. In plants, it supports vast networks, like in forests where roots connect via mycorrhizae, sharing warnings about pests.
Evolutionarily, these mechanisms likely arose to foster cooperation among cells, turning single-celled ancestors into integrated organisms. In bacteria, similar contact-dependent systems exist in biofilms, hinting at ancient origins.
In modern applications, understanding these could revolutionize medicine and agriculture. Gene therapies for connexin disorders or engineered plants with enhanced plasmodesmata defenses are on the horizon. Even in bioengineering, mimicking these junctions could create smarter materials or artificial tissues.
Challenges remain, like how climate change affects plant signaling or aging impacts animal junctions. Ongoing research promises deeper insights, potentially unlocking new treatments.
Wrapping Up
In summary, contact-dependent signaling through gap junctions and plasmodesmata weaves the fabric of life in animals and plants. These structures ensure cells don’t operate in isolation but as harmonious teams, driving everything from heartbeats to harvest yields. By appreciating their roles, we gain a deeper respect for the microscopic marvels that sustain us. As science advances, who knows what new discoveries will emerge from these tiny channels? The world of cell communication is vast and ever-evolving, inviting us to keep exploring.
Frequently Asked Questions
FAQ 1: What Are the Main Differences Between Gap Junctions in Animal Cells and Plasmodesmata in Plant Cells?
When comparing gap junctions in animals to plasmodesmata in plants, it’s clear these structures serve similar purposes in cell communication but have adapted to the unique needs of each kingdom. Both allow direct exchange of materials between cells, but their designs reflect the differences in cell walls and lifestyles. Animals, being mobile, need fast electrical signaling, while plants, rooted in place, focus on sharing nutrients across rigid barriers.
Structurally, gap junctions are made from proteins called connexins that form channels directly between plasma membranes. In contrast, plasmodesmata pierce through cell walls and include a central desmotubule from the endoplasmic reticulum, surrounded by a cytoplasmic sleeve. This makes plasmodesmata more like tunnels through walls, while gap junctions are simple pores between touching cells.
Functionally, gap junctions excel in quick ion and small molecule transfer, crucial for synchronized actions like heartbeats. Plasmodesmata handle a wider range, including larger proteins and RNA, aiding in plant growth and defense. Regulation differs too: gap junctions open and close via chemical signals like pH changes, whereas plasmodesmata use callose deposits to adjust size.
| Feature | Gap Junctions (Animal Cells) | Plasmodesmata (Plant Cells) |
|---|---|---|
| Composition | Six connexin proteins forming connexons that align between cells | Desmotubule from ER, cytoplasmic sleeve, and plasma membrane lining |
| Location | Between adjacent plasma membranes in tissues without cell walls | Traversing cell walls and membranes in walled plant cells |
| Permeability | Limited to small ions, metabolites (typically under 1 kDa) | Allows small molecules plus larger ones like proteins, RNA, and viruses when dilated |
| Regulation Mechanism | Gated by calcium, pH, or phosphorylation enzymes | Controlled by callose deposition and dilation proteins |
| Primary Role | Electrical coupling for rapid synchronization in muscles and nerves | Symplastic transport for nutrients, signals, and developmental cues |
| Evolutionary Adaptation | Suited for excitable, mobile organisms requiring fast responses | Designed for sessile, walled cells needing resource sharing over barriers |
| Examples in Action | Coordinating heart contractions or neuronal signals | Distributing sugars in phloem or signaling during root growth |
| Disease Implications | Linked to arrhythmias, deafness, and cancer progression | Involved in viral spread and developmental abnormalities in crops |
This table underscores how these structures, while analogous, are finely tuned for their environments, highlighting nature’s clever solutions to cellular connectivity.
FAQ 2: How Do Gap Junctions Support Heart Function and What Diseases Arise From Their Dysfunction?
Gap junctions play a starring role in keeping your heart beating steadily and strongly. In the cardiac muscle, these tiny channels connect neighboring cells, allowing ions like potassium and sodium to flow freely between them. This ion exchange creates electrical coupling, which means the electrical impulses that trigger contractions spread quickly and uniformly across the heart tissue. Without this seamless communication, the heart couldn’t pump blood efficiently, leading to irregular rhythms or even failure. It’s like a well-orchestrated symphony where each musician hears the conductor’s cues instantly through these junctions.
Beyond basic beating, gap junctions help in metabolic support during stress. When the heart works harder, such as during exercise, cells share nutrients and signaling molecules to maintain energy levels. Research shows that in healthy hearts, connexin 43, the main protein in these junctions, ensures this harmony. But when things go wrong, like in aging or after a heart attack, these junctions can remodel or close up, disrupting the flow and causing areas of the heart to beat out of sync.
Diseases linked to gap junction problems are serious and varied. For instance, arrhythmias occur when altered junctions slow down impulse propagation, leading to erratic heartbeats that can be life-threatening. Inherited conditions like arrhythmogenic cardiomyopathy stem from mutations in connexin genes, weakening the heart’s structure and electrical stability. In heart failure, reduced junction activity exacerbates damage by isolating cells, preventing them from supporting each other. Studies indicate that inflammation or ischemia can phosphorylate connexins, changing their function and contributing to these issues.
Therapeutically, scientists are exploring ways to fix these junctions. Drugs that stabilize connexin 43 or peptides mimicking junction proteins show promise in animal models, reducing arrhythmia risks post-infarction. Gene therapies to correct mutations are also on the horizon, aiming to restore normal communication. Understanding these mechanisms not only explains heart diseases but opens doors to targeted treatments, potentially saving countless lives by keeping the heart’s cellular network intact.
FAQ 3: How Do Plasmodesmata Help Plants Defend Against Viruses and Other Pathogens?
Plasmodesmata are like guarded gateways in plant cells, playing a dual role in both everyday communication and defense strategies. Normally, they allow the flow of nutrients and signals, but when a pathogen attacks, plants can tighten these channels to limit spread. This flexibility is key to plant immunity, where closing plasmodesmata prevents viruses from hopping from cell to cell.
In viral infections, many viruses exploit plasmodesmata by producing movement proteins that widen the channels, enabling their RNA or proteins to travel systemically. However, plants fight back by depositing callose, a carbohydrate that plugs the pores, effectively quarantining the infected area. This response is triggered by immune signals like salicylic acid, which ramps up callose synthase enzymes. For bacterial and fungal threats, plasmodesmata regulate the transport of defense molecules, such as reactive oxygen species or antimicrobial compounds, to frontline cells.
- Viral Containment Strategies: Plants induce callose buildup at plasmodesmata to block virus movement, as seen in tobacco mosaic virus infections where resistant varieties seal channels faster.
- Role in Systemic Acquired Resistance: Signals like small RNAs travel through open plasmodesmata to warn distant tissues, priming them for defense before the pathogen arrives.
- Fungal and Bacterial Defense: During invasions, plasmodesmata restrict effector proteins from pathogens, while allowing plant hormones like jasmonic acid to coordinate responses.
- Immune Protein Trafficking: Proteins like plasmodesmata-located proteins (PDLPs) act as sentinels, detecting threats and modulating channel permeability to enhance resistance.
- Pathogen Exploitation Tactics: Some fungi use hyphae to navigate plasmodesmata, but plants counter with chitin-triggered immunity that narrows these paths.
This battle at the cellular level shows how plasmodesmata are not just passive conduits but active players in plant survival, influencing everything from crop yields to ecosystem health.
FAQ 4: What Role Does Contact-Dependent Signaling Play in Animal Embryonic Development?
Contact-dependent signaling is essential during the early stages of animal embryonic development, where cells must coordinate to form tissues and organs. As the embryo grows from a single fertilized egg, cells divide and start touching each other, using surface molecules to exchange instructions. This direct communication helps determine cell fates, like whether a cell becomes part of the skin, nervous system, or muscles. For example, the Notch-Delta pathway is a classic case where a ligand on one cell binds to a receptor on a neighboring cell, triggering gene expression changes that guide differentiation.
In more detail, this signaling ensures proper patterning. During gastrulation, when the embryo folds into layers, contact signals prevent cells from straying and promote adhesion. Without it, embryos might develop abnormalities, such as neural tube defects in humans. Research highlights how calcium waves propagate through gap junctions in developing embryos, synchronizing cell movements and divisions. This is crucial in organogenesis, where heart precursors align and connect via connexins to establish rhythmic beating early on.
Furthermore, contact-dependent mechanisms regulate stem cell niches in embryos, maintaining a balance between proliferation and specialization. In fruit flies, for instance, germline stem cells rely on hub cell contacts to stay undifferentiated. Disruptions, like mutations in junction proteins, can lead to developmental disorders, emphasizing the precision required. As embryos mature, these signals integrate with other pathways, like hormonal ones, to sculpt the body plan. Overall, this form of signaling acts as the embryo’s blueprint, ensuring every cell knows its place and role in the grand design of life.
FAQ 5: How Are Gap Junctions Involved in Cancer Progression and What Potential Treatments Target Them?
Gap junctions have a complex relationship with cancer, sometimes acting as suppressors and other times as promoters depending on the stage and type. In early cancer, reduced gap junction activity often allows cells to ignore growth controls from neighbors, leading to uncontrolled proliferation. This loss of communication, frequently due to downregulated connexins, helps tumors evade apoptosis and spread. However, in advanced stages, gap junctions might reform to aid metastasis by enabling nutrient sharing in harsh tumor environments.
Treatments are exploring ways to manipulate these junctions. Restoring connexin expression through gene therapy could reinstate tumor suppression, as seen in studies where connexin 43 overexpression slows breast cancer growth.
- Inhibiting Metastasis: Drugs like rotigaptide enhance junction stability, potentially blocking cancer cell migration by maintaining contact inhibition.
- Targeting Hemichannels: Blocking unpaired connexin channels reduces inflammation that fuels tumors, with peptides showing promise in glioma models.
- Combination Therapies: Pairing junction modulators with chemotherapy improves drug delivery into tumors via reopened channels.
- Diagnostic Uses: Low connexin levels serve as biomarkers for aggressive cancers, guiding personalized treatments.
- Challenges in Dual Roles: Therapies must be context-specific, as boosting junctions in some cancers might promote dormancy or resistance.
This nuanced understanding is driving innovative approaches to harness gap junctions against cancer.
FAQ 6: What Are Some Key Examples of Contact-Dependent Signaling in the Nervous System?
Contact-dependent signaling in the nervous system ensures precise and rapid communication, from synapse formation to glial support. It’s vital for everything from reflex actions to learning.
| Example | Description | Key Components | Importance |
|---|---|---|---|
| Electrical Synapses | Direct ion flow between neurons via gap junctions for ultrafast signaling | Connexin 36 proteins forming channels | Enables synchronized firing in escape responses, like in fish startle reflexes |
| Glial-Neuronal Interactions | Astrocytes connect to neurons through junctions to regulate ion balance | Connexin 43 in astrocytic networks | Supports neuronal health by buffering potassium during high activity |
| Notch Signaling in Neurogenesis | Cell surface ligands activate receptors on adjacent cells to control differentiation | Delta-like ligands and Notch receptors | Guides stem cells to become neurons or glia during brain development |
| Ephrin-Eph Interactions | Bidirectional signaling at cell contacts for axon guidance | Ephrin ligands and Eph receptors | Directs growing axons to correct targets, preventing wiring errors |
| Immune Surveillance by Microglia | Contact with neurons to monitor and respond to damage | Various adhesion molecules | Allows quick inflammation control in response to injury or pathogens |
These examples illustrate how direct cell contacts fine-tune the nervous system’s complexity.
FAQ 7: How Have Gap Junctions and Plasmodesmata Evolved Across Different Organisms?
The evolution of gap junctions and plasmodesmata reflects the journey toward multicellularity, where cells needed ways to communicate directly. In ancient single-celled organisms, simple membrane pores might have sufficed for basic exchanges, but as life diversified, these structures became more sophisticated. Gap junctions, found in animals, likely originated from bacterial ancestors with similar channels, predating eukaryotic life by billions of years. Connexins evolved in metazoans to enable fast signaling in mobile bodies.
Plasmodesmata, unique to plants, trace back to green algae, where intercellular bridges helped in colonial living. As plants developed cell walls, these channels adapted to penetrate them, incorporating ER elements for versatility. Unlike gap junctions, plasmodesmata allow macromolecular transport, suiting sessile plants’ needs for resource sharing.
Convergently, both structures promote cooperation, but divergences highlight kingdom-specific pressures. Invertebrates have innexins instead of connexins, while advanced plants refined plasmodesmata for defense. This evolutionary tinkering underscores how direct signaling enabled complex life forms.
FAQ 8: What Therapeutic Strategies Are Being Developed to Target Gap Junctions in Diseases?
Therapeutic targeting of gap junctions is gaining traction as researchers uncover their roles in various conditions. Strategies focus on modulating connexin function to restore normal communication or block harmful effects.
- Peptide Mimetics: Short peptides mimicking connexin sequences prevent channel closure in ischemia, protecting heart tissue post-attack.
- Gene Therapy: Delivering connexin genes to deficient cells, like in skin disorders, to rebuild junctions and improve healing.
- Small Molecule Modulators: Compounds like danegaptide enhance junction opening in arrhythmias, stabilizing rhythms in clinical trials.
- Antibody-Based Approaches: Antibodies targeting specific connexins in cancer to disrupt tumor communication without affecting healthy tissues.
- Hemichannel Blockers: Drugs inhibiting unpaired connexins reduce inflammation in neurological diseases like epilepsy.
These innovations promise personalized treatments, balancing junction activity for better outcomes.
FAQ 9: How Do Plasmodesmata Facilitate Nutrient Transport and Distribution in Plants?
Plasmodesmata are crucial for nutrient transport in plants, creating a symplastic highway that bypasses cell membranes for efficient distribution. In leaves, where photosynthesis occurs, sugars like sucrose move through these channels to phloem tissues, then travel to roots or fruits. This flow, driven by pressure gradients, ensures energy reaches non-photosynthetic parts without energy loss from repeated membrane crossings.
Regulation adds sophistication: during growth, plasmodesmata dilate to allow larger nutrient complexes, while in stress, they constrict to conserve resources. In roots, they enable mineral uptake from soil, sharing ions like potassium across cells. Hormones like auxin also hitch rides, influencing development.
In agriculture, understanding this enhances crop efficiency. Mutants with altered plasmodesmata show stunted growth, highlighting their role in yield. Recent discoveries link pectin modifications to channel openness, potentially boosting transport in faster-growing varieties.
FAQ 10: Why Is Contact-Dependent Signaling Important for Immune Responses in Animals?
Contact-dependent signaling is a cornerstone of immune responses, enabling precise coordination among cells to fight invaders. In T cell activation, for example, antigen-presenting cells touch T cells via receptors, triggering cytokine release and proliferation to mount an attack.
This direct interaction ensures specificity, preventing unnecessary inflammation. NK cells use contacts to detect and kill infected cells, while gap junctions propagate alerts like DNA signals across immune networks. In tissues, it guides migration, with chemokines drawing cells together for collaborative defense.
Disruptions lead to autoimmunity or weak responses, as seen in junction defects. This signaling integrates with other pathways, amplifying the body’s defenses efficiently.
FAQ 11: How Do Gap Junctions Contribute to Wound Healing in Animals?
Gap junctions are like the unsung heroes of wound healing in animals, orchestrating a complex dance of cellular teamwork to repair damaged tissue. These tiny channels act as direct lines of communication, allowing cells to share critical signals and resources instantly. Understanding their role sheds light on how our bodies mend cuts, burns, or deeper injuries, and even points to exciting possibilities for improving healing in medical treatments.
Here’s a detailed look at how gap junctions make this happen:
- Role as Communication Links: Gap junctions serve as vital communication channels in animals, enabling cells to coordinate their response to injury during the wound healing process.
- Coordination of Cellular Response: When skin or tissue is damaged, gap junctions help cells at the wound site work together to close the gap, reduce inflammation, and regenerate new tissue.
- Sharing of Signaling Molecules: These junctions facilitate the rapid exchange of ions, metabolites, and signaling molecules such as cyclic AMP and IP3, which synchronize critical cell behaviors like migration and proliferation.
- Initial Response to Injury: In the early stages of wound healing, gap junctions may decrease in number or activity to isolate the damaged area, preventing the spread of harmful signals or substances.
- Reformation for Collective Movement: As healing progresses, gap junctions reform to support collective cell movement, allowing cells at the wound edge to pull together, resembling a team closing a drawstring bag.
- Importance of Connexin 43: Research highlights that specific connexins, particularly connexin 43, play a significant role in wound healing.
- Regulation of Connexin 43: In normal healing, connexin 43 expression temporarily decreases at the wound front, which speeds up cell migration and reduces scar formation.
- Consequences of Dysregulation: If connexin 43 regulation fails, it can lead to chronic wounds or excessive scarring, impairing the healing process.
- Evidence from Mouse Studies: Studies on mice with connexin 43 deficiencies show faster wound closure and reduced inflammation, indicating potential therapeutic targets.
- Application to Human Treatments: Insights from these studies suggest that targeting connexin 43 could improve treatments for slow-healing wounds, such as diabetic ulcers in humans.
- Immune Response Coordination: Gap junctions enable keratinocytes and fibroblasts to exchange signaling molecules, alerting immune cells like macrophages to clear debris from the wound site.
- Repair in Deeper Tissues: Beyond skin, gap junctions aid in repairing deeper tissues like fascia, where connexin 43 drives mobilization and patch repair to restore structural integrity.
- Role of Hemichannels: Hemichannels, which are unpaired halves of gap junctions, release ATP to signal distress, aiding in the early response to injury.
- Risk of Prolonged Inflammation: Uncontrolled hemichannel activity can prolong inflammation, potentially delaying the healing process.
- Emerging Therapies: Therapies using peptides to modulate connexin function are being developed to fine-tune gap junction activity, aiming to enhance outcomes in surgical recovery or burn treatment.
- Dynamic Role in Healing: Overall, gap junctions demonstrate a dynamic role, shifting from acting as barriers to isolate damage to serving as bridges that facilitate coordinated healing.
FAQ 12: What Is the Impact of Environmental Stress on Plasmodesmata Function in Plants?
Environmental stress profoundly affects plasmodesmata, the channels that connect plant cells and enable resource sharing. Under conditions like drought, heavy metal exposure, or pathogen attacks, plants often alter these channels to protect themselves, either by closing them to isolate stressed areas or opening them to distribute defense signals. This adaptability helps plants survive harsh conditions but can also disrupt normal growth if prolonged.
- Drought and Water Stress: Abscisic acid, a stress hormone, triggers plasmodesmata to reduce density or close via callose buildup, conserving water by limiting symplastic flow.
- Heavy Metal Toxicity: Exposure to metals like cadmium prompts quick changes in plasmodesmatal permeability, often narrowing channels to prevent toxin spread while adjusting cell communication for detoxification.
- Cold or Heat Stress: Low temperatures induce closure through reactive oxygen species signaling, protecting cells from freezing damage, whereas heat might dilate them for better nutrient redistribution.
- Pathogen Invasion: During biotic stress, plasmodesmata act as gates, closing to contain viruses but allowing immune signals like salicylic acid to pass, enhancing systemic resistance.
- Salinity Effects: High salt levels lead to callose deposition, restricting ion movement and helping maintain cellular balance, though chronic stress can impair development.
- Overall Coordination: Stress signals integrate through plasmodesmata, linking ER and plasma membrane for holistic responses, underscoring their role as unconventional contact sites.
These responses showcase how plasmodesmata serve as dynamic regulators, balancing isolation and connectivity to bolster plant resilience.
FAQ 13: How Does Contact-Dependent Signaling Compare to Other Cell Signaling Methods?
Contact-dependent signaling stands out for requiring physical touch between cells, differing from methods that send signals over distances. This direct approach ensures precise, local communication, ideal for synchronized activities like tissue development. In contrast, other methods like paracrine or endocrine signaling rely on diffusible molecules, allowing broader influence but with less specificity.
| Signaling Type | Description | Range | Key Molecules | Examples | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Contact-Dependent | Cells communicate via direct membrane contact or channels like gap junctions and plasmodesmata | Immediate neighbors | Receptors, ligands, ions through channels | Notch signaling in development, ion flow in heart muscle | High precision, rapid synchronization | Limited to touching cells, vulnerable to physical disruption |
| Paracrine | Signals diffuse to nearby cells through extracellular fluid | Local, short-range | Growth factors, cytokines | Wound healing signals from injured cells to neighbors | Coordinates local responses without systemic effects | Can be diluted or intercepted |
| Endocrine | Hormones travel via bloodstream to distant targets | Long-range, systemic | Insulin, thyroid hormones | Glucose regulation by pancreas affecting whole body | Affects multiple organs simultaneously | Slower, requires circulation |
| Synaptic | Neurons release neurotransmitters across synapses | Very short, but targeted | Acetylcholine, dopamine | Nerve impulses in muscles or brain circuits | Extremely fast and specific | Limited to nervous system, energy-intensive |
| Autocrine | Cell signals itself with its own secretions | Self | Interleukin-2 in immune cells | Cancer cells promoting own growth | Self-regulation | Can lead to uncontrolled loops |
| Juxtacrine | Membrane-bound signals interact with adjacent cell receptors | Immediate contact | Ephrins in axon guidance | Tissue boundary formation | Stable, no diffusion needed | Requires stable cell positions |
This comparison illustrates how each method suits different biological needs, with contact-dependent offering intimate control while others provide flexibility.
FAQ 14: What Techniques Are Used to Study Gap Junctions and Plasmodesmata?
Studying gap junctions and plasmodesmata involves a mix of imaging, electrophysiological, and molecular techniques to uncover their structure, function, and dynamics. Researchers often start with microscopy methods, like electron microscopy, to visualize these tiny channels at high resolution. For instance, transmission electron microscopy reveals the cylindrical connexons in gap junctions or the desmotubule in plasmodesmata, providing snapshots of their architecture. Fluorescence microscopy, enhanced by dyes or GFP-tagged proteins, tracks their location and permeability in living cells, showing how they open or close in response to stimuli.
Electrophysiological approaches measure functional aspects. Paired recordings from connected cells assess electrical coupling, quantifying how ions flow through gap junctions. For plasmodesmata, microinjection of fluorescent tracers like Lucifer yellow evaluates symplastic transport, revealing selectivity for molecule size. Tracer coupling combined with network analysis maps connectivity in tissues, helping understand roles in development or stress.
Molecular tools delve into genetics and biochemistry. Knockout models, such as connexin-deficient mice or plasmodesmata protein mutants in plants, expose functional impacts. Western blotting and immunoprecipitation identify interacting proteins, while CRISPR editing targets specific components for precise studies. Advanced methods like super-resolution microscopy or cryo-electron tomography offer 3D views, advancing our grasp of these complex structures.
FAQ 15: How Do Plasmodesmata Influence Plant Reproduction and Development?
Plasmodesmata are vital in shaping plant reproduction and development by enabling the flow of signals and nutrients that guide growth patterns and reproductive success. During embryogenesis, they facilitate the movement of transcription factors, ensuring cells differentiate correctly into roots, shoots, or flowers.
- Embryo Patterning: Transcription factors like SHORT-ROOT move through plasmodesmata to define tissue layers, with restrictions creating boundaries for proper organ formation.
- Leaf Vein Development: They regulate auxin transport, influencing vein patterns essential for nutrient distribution and overall plant architecture.
- Floral Induction: Signals such as florigen proteins travel via these channels to synchronize flowering across the plant, impacting seed production.
- Pollen Tube Guidance: In reproduction, plasmodesmata aid in gamete communication, ensuring fertilization by sharing molecular cues.
- Fruit Ripening: During fruit development, they control sugar unloading in sinks, affecting size and quality.
- Stress Adaptation in Reproduction: Under pressure, turgor changes modulate plasmodesmata, balancing growth and survival for viable offspring.
This interconnectedness underscores plasmodesmata’s role in orchestrating life’s cycle in plants.
FAQ 16: Which Neurological Disorders Are Associated with Gap Junction Dysfunction?
Gap junction issues contribute to various neurological disorders by disrupting cellular communication in the brain and nerves. Mutations or altered expression of connexins lead to impaired ion balance, inflammation, and neuronal death.
| Disorder | Description | Affected Connexins | Symptoms | Mechanisms |
|---|---|---|---|---|
| Charcot-Marie-Tooth Disease (CMT1X) | Inherited neuropathy affecting peripheral nerves | GJB1 (Connexin 32) | Muscle weakness, sensory loss | Disrupted myelin sheath signaling |
| Pelizaeus-Merzbacher-Like Disease (PMLD) | Dysmyelinating disorder in central nervous system | GJC2 (Connexin 47) | Nystagmus, motor delays | Oligodendrocyte gap junction failure |
| Epilepsy | Seizure disorders with hypersynchronized activity | Various, e.g., Connexin 36 | Convulsions, loss of consciousness | Altered astrocytic and neuronal coupling |
| Alzheimer’s Disease | Neurodegenerative with cognitive decline | Connexin 43 in astrocytes | Memory loss, confusion | Increased hemichannel activity promoting inflammation |
| Parkinson’s Disease | Movement disorder with dopamine loss | Multiple connexins | Tremors, rigidity | Disrupted glial-neuronal junctions exacerbating neurodegeneration |
| Brain Ischemia/Stroke | Damage from reduced blood flow | Connexin 43 | Paralysis, speech issues | Gap junction remodeling leading to cell death spread |
| Major Depressive Disorder (MDD) | Mood disorder affecting prefrontal cortex | Astrocytic connexins | Persistent sadness, anhedonia | Reduced gap junction coupling in astrocytes |
This table highlights the broad impact of gap junction problems on brain health.
FAQ 17: What Agricultural Applications Arise from Understanding Plasmodesmata?
Understanding plasmodesmata opens exciting avenues in agriculture, from boosting crop yields to enhancing disease resistance. These channels control nutrient flow, so manipulating them could optimize sugar distribution in fruits, leading to larger, sweeter produce. For instance, research on phloem unloading via plasmodesmata in tomatoes suggests genetic tweaks could improve fruit size without compromising quality. In staple crops like rice or wheat, better symplastic transport might increase biomass, addressing food security challenges.
Disease management is another key area. Viruses often hijack plasmodesmata to spread, so engineering plants with regulated channels—perhaps through callose-modifying genes—could create resistant varieties. Studies on receptor-like proteins at plasmodesmata show promise for blocking pathogens while maintaining normal signaling. This could reduce pesticide use, promoting sustainable farming.
Nutrient efficiency ties in too. By fine-tuning plasmodesmata, plants might better absorb and distribute fertilizers, minimizing runoff and environmental harm. Emerging biotech, like CRISPR edits to plasmodesmata proteins, aims at drought-tolerant crops that close channels during stress to save water. Overall, this knowledge could revolutionize agriculture, making crops more resilient and productive.
FAQ 18: How Conserved Are Contact-Dependent Signaling Mechanisms Across Species?
Contact-dependent signaling mechanisms show remarkable conservation, reflecting their ancient origins in multicellular life. Gap junctions in animals and plasmodesmata in plants, though structurally different, serve analogous roles, with bacterial precursors hinting at billions of years of evolution.
- Bacterial Ancestors: Prokaryotic channels resemble gap junctions, providing a framework that predates eukaryotes.
- Invertebrate Variations: Innexins in worms and flies function like connexins, regulating development and longevity.
- Vertebrate Consistency: Connexins are highly conserved from fish to mammals, essential for heart and brain function.
- Plant Lineage: Plasmodesmata proteins trace to algae, with conservation in mosses and angiosperms for stress responses.
- Cross-Kingdom Parallels: Both systems use callose-like regulators, showing convergent evolution for intercellular exchange.
- Developmental Roles: Conserved in embryogenesis, delimiting cell fates across species.
This conservation underscores their fundamental importance in life’s complexity.
FAQ 19: What Are the Future Directions in Research on Contact-Dependent Signaling?
Future research on contact-dependent signaling promises to unravel more about these vital cellular links, focusing on high-resolution imaging and genetic tools. Scientists aim to build detailed 3D models of gap junctions and plasmodesmata using cryo-electron microscopy, revealing how proteins interact dynamically. This could clarify permeability controls, aiding in designing drugs for junction-related diseases.
Integration with other cellular systems is a hot area. Exploring how plasmodesmata act as ER-plasma membrane contact sites might explain nutrient exchange and stress signaling. In animals, studying hemichannels’ roles in inflammation could lead to therapies for neurodegeneration.
Biotech applications loom large, like engineering plasmodesmata for better crop traits or using CRISPR to fix connexin mutations. Investigating redox signaling through these channels may uncover new roles in development and aging. Collaborative efforts across disciplines will drive these advances, potentially transforming medicine and agriculture.
FAQ 20: What Role Do Gap Junctions and Plasmodesmata Play in Aging and Longevity?
Gap junctions and plasmodesmata influence aging by modulating cellular communication, with changes over time affecting tissue function and lifespan. In animals, reduced gap junction coupling in astrocytes contributes to cognitive decline, while in plants, altered plasmodesmata impact stress resilience.
- Animal Nervous System: In worms, innexin genes regulate lifespan, with mutations extending or shortening it by affecting neuronal signaling.
- Brain Aging: Decreased connexin 43 in aging brains leads to inflammation and neuronal loss, linked to disorders like Alzheimer’s.
- Tissue Maintenance: Pathological remodeling, like trafficking errors, accelerates aging in organs like the heart.
- Plant Longevity: Plasmodesmata help distribute anti-aging signals, with stress-induced closures promoting survival in perennials.
- Cross-Species Insights: Conserved mechanisms suggest targeting junctions could enhance longevity, as seen in model organisms.
- Therapeutic Potential: Boosting junction function might mitigate age-related decline, opening avenues for anti-aging interventions.
Acknowledgement
The Examsmeta.com expresses its gratitude to the various scientific resources that provided invaluable insights for the article “Understanding Contact-Dependent Signaling: How Cells Communicate Through Direct Contact in Animals and Plants.” Their comprehensive and reliable information on cellular biology, gap junctions, and plasmodesmata greatly enriched our understanding and presentation of this complex topic.
Specifically, acknowledges the contributions of Nature (www.nature.com) for its extensive peer-reviewed articles on cellular communication mechanisms, ScienceDirect (www.sciencedirect.com) for its detailed studies on connexins and plant signaling pathways, and PubMed (pubmed.ncbi.nlm.nih.gov) for its vast repository of biomedical research that informed our discussions on disease implications. Their authoritative content ensured the accuracy and depth of our article.

