DNA, the blueprint of life, holds the instructions for building and maintaining every living organism on Earth. Yet, one of the most fascinating aspects of this molecule is how it manages to fit inside the minuscule confines of a cell’s nucleus. Imagine trying to stuff a 2-meter-long thread into a space smaller than a grain of sand, that’s the challenge cells face with DNA. This process, known as DNA packaging, is not just a matter of space-saving; it’s a sophisticated mechanism that influences gene expression, cell function, and even evolution.
In this comprehensive article, we’ll dive deep into what DNA packaging is, how it works in different types of cells, the proteins involved, and why it’s crucial for life. We’ll explore historical insights, modern understandings, and real-world implications, drawing from established scientific knowledge to paint a complete picture.
Table of Contents
The story of DNA packaging begins with the groundbreaking discovery of DNA’s structure itself. In the early 1950s, scientists were racing to unravel the mystery of how genetic information was stored and passed on.
James Watson and Francis Crick, building on the work of Rosalind Franklin and Maurice Wilkins, proposed the double-helix model of DNA in 1953. This model showed DNA as two intertwined strands, like a twisted ladder, with bases pairing up in the middle. Their work, published in a seminal paper, laid the foundation for understanding how DNA could replicate and function. But it also raised questions: how does such a long molecule fit into cells? It turns out, DNA doesn’t just float around; it’s meticulously packaged with proteins, a concept that emerged from further research into chromosomes and histones.
What is DNA?
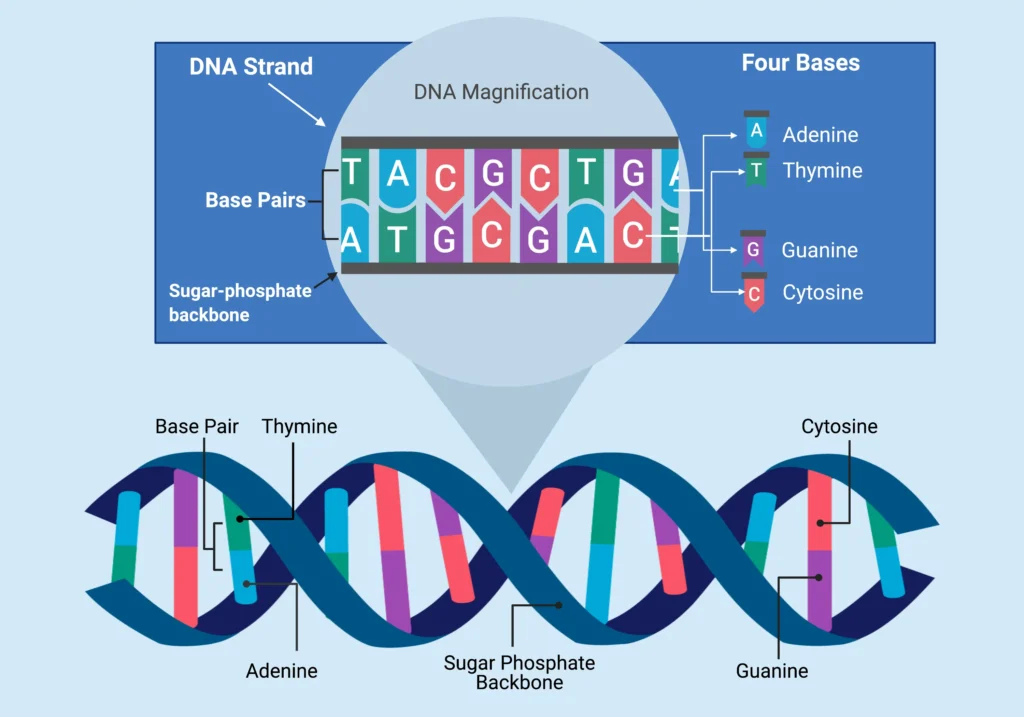
Before delving into packaging, let’s clarify what DNA is. Deoxyribonucleic acid (DNA) is a long, chain-like molecule made up of nucleotides, each consisting of a sugar, a phosphate group, and a nitrogenous base (adenine, thymine, cytosine, or guanine). These nucleotides link together to form strands, and in most organisms, two strands pair up via hydrogen bonds between complementary bases: adenine with thymine, cytosine with guanine.
DNA serves as the hereditary material, carrying genes that code for proteins essential to life. It’s found in nearly every cell, from bacteria to humans, and even in many viruses. In humans, if you stretched out all the DNA from one cell, it would measure about 2 meters long. Multiply that by the trillions of cells in your body, and you’d have enough DNA to reach the sun and back multiple times. This sheer length underscores the need for efficient packaging.
The Structure of DNA
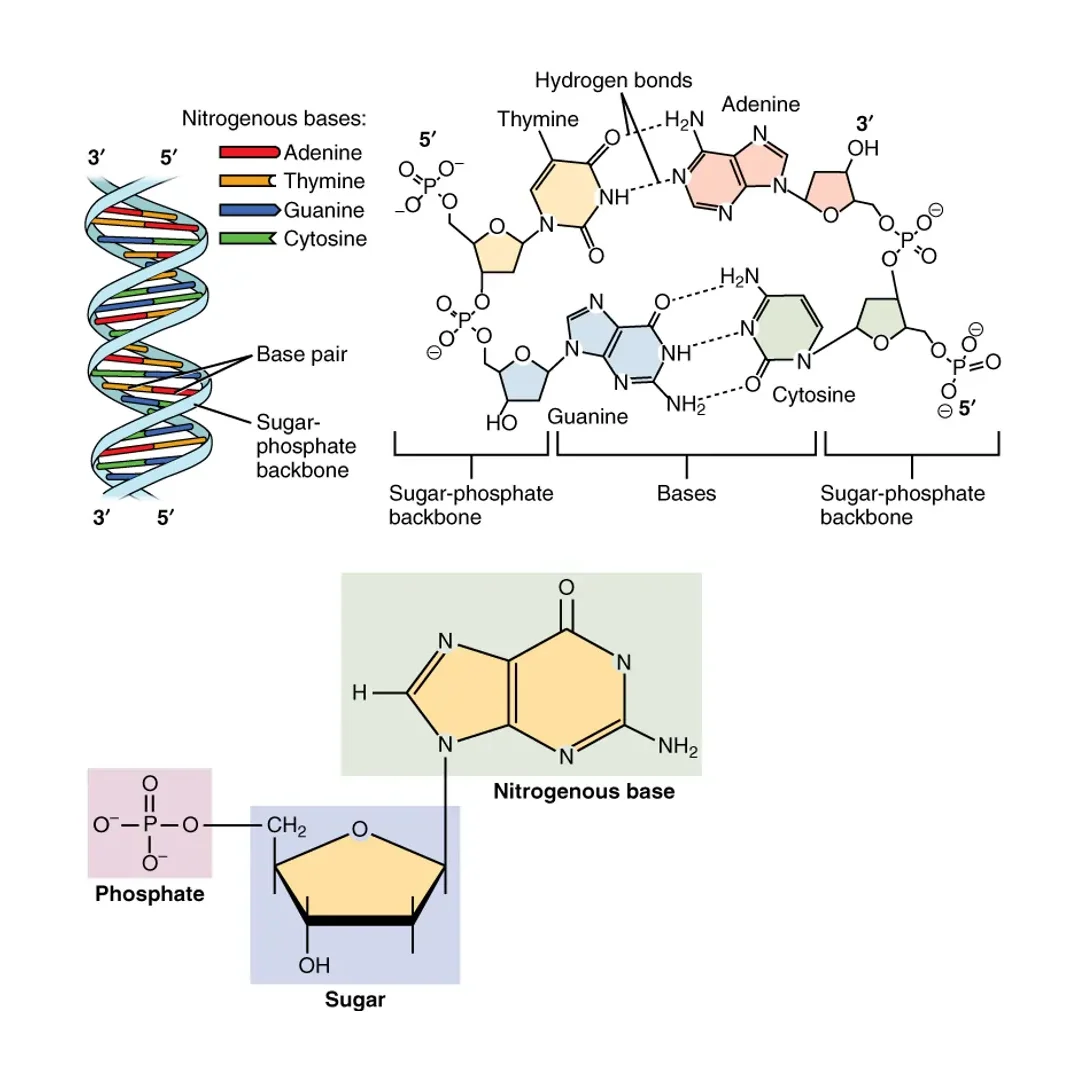
The structure of DNA, as proposed by Watson and Crick, is a right-handed double helix. Each strand runs anti-parallel to the other, meaning one goes 5′ to 3′ while the other is 3′ to 5′. The helix has a pitch of about 3.4 nanometers per turn, accommodating roughly 10 base pairs per twist. The backbone, composed of sugar-phosphate links, is negatively charged due to the phosphate groups, which plays a key role in how DNA interacts with packaging proteins.
This negative charge makes DNA repel itself, but cells overcome this by using positively charged proteins to bind and neutralize it. The double helix is stable yet flexible, allowing it to twist, bend, and compact as needed. In viruses, DNA can be linear or circular, but in cells, it’s typically linear in eukaryotes and circular in prokaryotes.
What is DNA Packaging?
DNA packaging is the intricate process by which cells compact their genetic material to fit within the limited space of the nucleus or nucleoid region while still allowing access for essential functions like replication and transcription. Think of it as folding a long rope into a neat ball without knots that prevent you from pulling out sections when needed.
In simple terms, DNA packaging transforms a sprawling molecule into a highly organized structure. Without it, DNA would tangle hopelessly, leading to cellular chaos. This process involves wrapping DNA around proteins, forming loops, and further condensing into higher-order structures. It’s dynamic, changing based on the cell’s needs, such as loosening during gene expression or tightening during cell division.
DNA Packaging in Eukaryotes
Eukaryotic cells, like those in plants, animals, and fungi, have a well-defined nucleus where DNA resides. Here, DNA is packaged into chromatin, a complex of DNA and proteins. The primary proteins are histones, which form the core around which DNA winds.
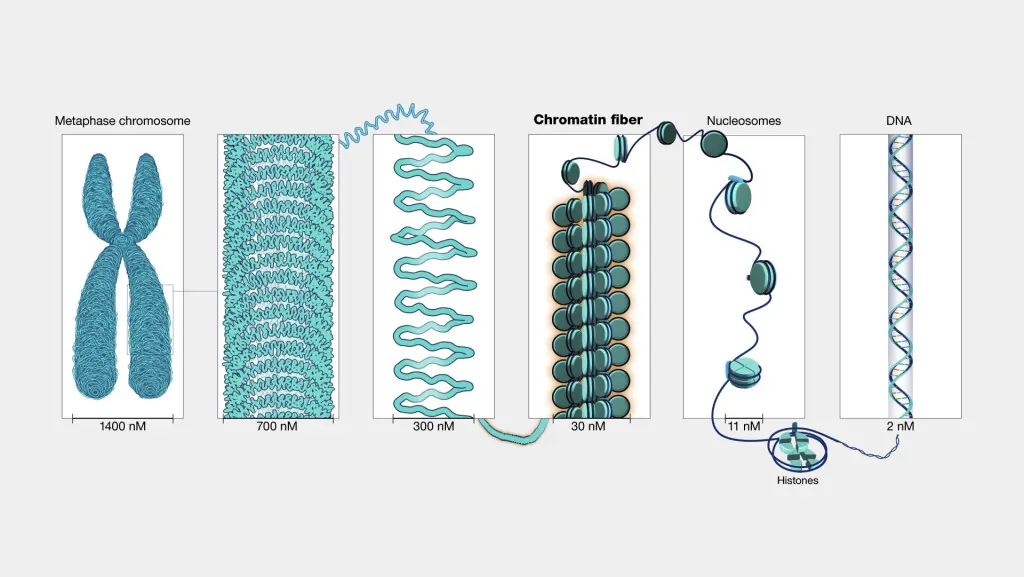
The basic unit is the nucleosome, where about 147 base pairs of DNA wrap around an octamer of histones (two each of H2A, H2B, H3, and H4). This looks like beads on a string under a microscope. Nucleosomes then fold into a 30-nanometer fiber, often called a solenoid, though recent studies suggest it might be more irregular. These fibers loop around scaffold proteins to form even larger structures, culminating in chromosomes during mitosis.
Eukaryotic packaging achieves massive compaction: human DNA is condensed about 10,000-fold to fit into the nucleus. This isn’t static; regions called euchromatin are loosely packed for active genes, while heterochromatin is tightly packed for inactive ones.
Role of Histones
Histones are the stars of DNA packaging in eukaryotes. These small, positively charged proteins bind to the negatively charged DNA backbone, neutralizing repulsions and enabling tight wrapping. There are five main types: H1 (linker histone) and the core histones H2A, H2B, H3, and H4.
Core histones form the octamer, with DNA wrapping 1.65 times around it to make a nucleosome. Linker histones like H1 seal the DNA entry and exit points, stabilizing the structure and aiding higher-order folding. Histones are rich in lysine and arginine residues, which provide the positive charge.
Beyond packaging, histones regulate access to DNA. For example, during transcription, histones must be temporarily displaced to allow RNA polymerase to read the genes.
Here’s a detailed table outlining the types of histones and their functions:
| Histone Type | Composition | Function | Key Features |
|---|---|---|---|
| H2A | One of the core histones, forms dimers with H2B | Part of the nucleosome core, involved in DNA wrapping and chromatin compaction | Can be modified by ubiquitination, affecting gene silencing |
| H2B | Pairs with H2A in dimers | Contributes to nucleosome stability and interacts with other chromatin proteins | Phosphorylation linked to apoptosis and cell death regulation |
| H3 | Forms dimers with H4, central to the octamer | Key in epigenetic marking, influences gene expression | Methylation at lysine 9 (H3K9me) promotes heterochromatin formation |
| H4 | Pairs with H3 | Stabilizes the histone octamer, highly conserved across species | Acetylation loosens chromatin for transcription |
| H1 | Linker histone, not part of the core octamer | Binds linker DNA between nucleosomes, promotes higher-order chromatin folding | Removal allows for gene activation and transcription |
This table highlights how each histone contributes uniquely to packaging and regulation.
Histone Modifications and Epigenetics
Histones aren’t just passive spools; they undergo chemical modifications that fine-tune DNA packaging and gene activity. This is the realm of epigenetics, where changes in gene expression occur without altering the DNA sequence itself.
Common modifications include:
- Acetylation: Adding acetyl groups to lysine residues reduces the positive charge, loosening DNA-histone bonds and promoting gene activation.
- Methylation: Can either activate or repress genes depending on the site; for instance, H3K4 methylation activates, while H3K27 represses.
- Phosphorylation: Often signals DNA damage repair or chromatin condensation during mitosis.
- Ubiquitination: Marks histones for degradation or alters chromatin structure.
These modifications form a “histone code” that chromatin readers interpret to control processes like transcription and replication. Enzymes like histone acetyltransferases (HATs) add acetyl groups, while histone deacetylases (HDACs) remove them. Histone methyltransferases (HMTs) add methyl groups.
For example, in cancer cells, abnormal histone methylation can silence tumor suppressor genes, leading to uncontrolled growth. In development, these modifications help differentiate stem cells into specialized types, like turning a pluripotent cell into a neuron by activating brain-specific genes while silencing others.
Recent research has uncovered novel modifications like lactylation and crotonylation, which link metabolism to epigenetics. These changes can be inherited across cell divisions, influencing traits without genetic mutations.
To illustrate the diversity, consider this extensive table of common histone modifications:
| Modification Type | Target Histone/Site | Effect on Chromatin | Biological Role | Example in Disease |
|---|---|---|---|---|
| Acetylation | H3K9, H4K16 | Loosens chromatin (euchromatin formation) | Promotes transcription and replication | Overacetylation in neurodegenerative diseases like Alzheimer’s |
| Methylation (mono/di/tri) | H3K4me3 | Activates genes | Enhances promoter activity | Hypermethylation in cancer, silencing tumor suppressors |
| Methylation | H3K27me3 | Represses genes | Maintains heterochromatin | Linked to developmental disorders like Rett syndrome |
| Phosphorylation | H3S10 | Condenses chromatin during mitosis | Aids in chromosome segregation | Defects in DNA damage response leading to genomic instability |
| Ubiquitination | H2BK120 | Alters nucleosome stability | Involved in DNA repair and transcription elongation | Mutations associated with lymphomas |
| SUMOylation | Various histones | Inhibits transcription | Stress response and genome stability | Dysregulation in viral infections |
| Crotonylation | H3K18 | Activates genes | Links to metabolic states | Emerging role in diabetes and obesity |
| Lactylation | H3K18 | Promotes gene expression | Connects lactate metabolism to epigenetics | Elevated in tumor microenvironments |
This table shows how modifications interplay to regulate the genome dynamically.
Levels of DNA Packaging
DNA packaging occurs in hierarchical levels, each building on the previous for greater compaction.
- First level: Nucleosome. DNA wraps around histone octamers, reducing length by about 7-fold.
- Second level: 30-nm fiber. Nucleosomes coil into a solenoid or zigzag structure, compacting another 6-fold.
- Third level: Loops and scaffolds. Fibers form loops attached to a protein scaffold, achieving 100-fold more compaction.
- Fourth level: Chromosomes. During cell division, loops condense into visible chromosomes, with total compaction up to 10,000-fold.
In interphase, chromatin is less condensed, allowing gene access. During mitosis, it’s maximally packed for equal distribution to daughter cells.
For a visual breakdown, here’s a structured table of packaging levels:
| Packaging Level | Structure Description | Compaction Factor | Key Components | Functional Importance |
|---|---|---|---|---|
| Naked DNA | Double helix strand | 1 (baseline) | DNA only | Theoretical; not found in cells due to length |
| Nucleosome | DNA wrapped around histone octamer (147 bp per nucleosome) | ~7-fold | Core histones (H2A, H2B, H3, H4) | Basic unit; protects DNA and regulates access |
| 30-nm Fiber (Solenoid) | Coiled nucleosomes with linker DNA | ~40-fold total | Linker histone H1 | Forms chromatin fiber; allows for euchromatin/heterochromatin distinction |
| Looped Domains | Fibers looped on nuclear matrix or scaffold proteins | ~800-fold total | Scaffold proteins like topoisomerase II | Organizes genes into functional domains; facilitates long-range interactions |
| Metaphase Chromosome | Highly condensed loops during mitosis | ~10,000-fold total | Condensins and cohesins | Ensures accurate segregation; visible under microscope |
This progression ensures both compactness and functionality.
DNA Packaging in Prokaryotes
Prokaryotic cells, such as bacteria, lack a nucleus, so their DNA is packaged in a region called the nucleoid. Unlike eukaryotes, they don’t use histones; instead, they rely on supercoiling and nucleoid-associated proteins (NAPs) like HU, IHF, and FIS.
DNA in prokaryotes is typically circular, which helps in compaction. Enzymes like topoisomerases introduce negative supercoils, twisting the helix to make it more compact. NAPs bind DNA, bending or looping it to organize the genome into domains.
For instance, in E. coli, the 4.6 million base pair genome is compacted into a nucleoid about 1 micrometer across. This packaging is less complex but highly efficient, allowing rapid replication in fast-growing bacteria.
Differences Between Prokaryotic and Eukaryotic DNA Packaging
While both achieve compaction, prokaryotic and eukaryotic packaging differ significantly.
- Location: Eukaryotes package in the nucleus; prokaryotes in the cytoplasm’s nucleoid.
- Proteins: Eukaryotes use histones; prokaryotes use NAPs and no true histones (though archaea have histone-like proteins).
- Structure: Eukaryotic DNA is linear and forms chromosomes; prokaryotic is circular and supercoiled.
- Compaction Level: Eukaryotes achieve higher compaction due to larger genomes.
- Regulation: Eukaryotic packaging ties deeply to epigenetics; prokaryotic is more about structural organization.
A comparison table for clarity:
| Aspect | Prokaryotic Packaging | Eukaryotic Packaging |
|---|---|---|
| Genome Shape | Mostly circular | Linear |
| Primary Proteins | NAPs (e.g., HU, FIS) | Histones (core and linker) |
| Basic Unit | Supercoiled loops | Nucleosomes |
| Compaction Mechanism | Supercoiling, bending | Wrapping, folding, looping |
| Location | Nucleoid in cytoplasm | Nucleus |
| Epigenetic Role | Limited | Extensive via modifications |
| Example Organism | Bacteria like E. coli | Humans, yeast |
| Genome Size Accommodation | Smaller genomes (e.g., 4 Mb) | Larger genomes (e.g., 3 Gb in humans) |
These differences reflect evolutionary adaptations.
Why is DNA Packaging Required?
The primary reason is space: a human nucleus is only 6 micrometers wide, but unraveled DNA is 2 meters long. Packaging compresses it dramatically. But it’s more than that; it protects DNA from damage, prevents tangling, and regulates gene access.
Without proper packaging, DNA replication and repair would be inefficient. It also allows cells to turn genes on or off as needed, like activating immune genes during infection.
Benefits of DNA Packaging
DNA packaging offers several advantages:
- Space Efficiency: Fits vast genetic material into tiny spaces.
- Gene Regulation: Distinguishes active (euchromatin) from inactive (heterochromatin) regions.
- Protection: Shields DNA from nucleases and mechanical stress.
- Replication Control: Ensures accurate copying during cell division.
- Evolutionary Flexibility: Allows complex organisms with large genomes.
For example, in sperm cells, DNA is ultra-compacted with protamines instead of histones for streamlined delivery.
Diseases Related to Faulty DNA Packaging
When packaging goes wrong, diseases can arise. Mutations in histone genes or modifying enzymes disrupt chromatin, leading to issues.
- Cancer: Aberrant histone modifications silence tumor suppressors or activate oncogenes.
- Muscular Dystrophy: Mutations in DNA packaging genes like LMNA cause facioscapulohumeral muscular dystrophy.
- Neurodevelopmental Disorders: Rett syndrome involves faulty methylation, affecting brain development.
- Aging and Neurodegeneration: Accumulated errors in packaging contribute to Alzheimer’s, where hyperphosphorylated histones impair repair.
- Multiple Myeloma: Changes in DNA repair and packaging genes increase risk.
Therapies targeting HDAC inhibitors are being developed for cancer, showing how understanding packaging can lead to treatments.
Conclusion
DNA packaging is a testament to nature’s ingenuity, transforming a simple molecule into a dynamic, regulated system essential for life. From the double helix discovered by Watson and Crick to the epigenetic layers uncovered today, it continues to reveal secrets about health, disease, and inheritance. As research advances, we may unlock new ways to manipulate packaging for medical breakthroughs, ensuring this microscopic marvel keeps inspiring generations.
Read These Articles in Detail
- The Cell: The Fundamental Building Block of Life
- Prokaryotic Cells vs. Eukaryotic Cells: A Detailed Exploration
- Prokaryotic Cells: Definition, Anatomy, Functions, and Their Importance
- Eukaryotic Cells Explained: Definition, Structure, Functions, and Importance
- The Cell Membrane: Structure, Functions, and Evolutionary Significance
- The Cell Wall: Structure, Composition, Functions, and Evolutionary Significance
- Endomembrane System: A Detailed Exploration of the Cellular Machinery of Life
- Mitochondria: A Comprehensive Guide to Their Structure and Functions
- The Cell Envelope: The Protective Shield of Prokaryotic Cells
- Ribosomes: Definition, Structure, and Functions
- Endoplasmic Reticulum: Structure, Types, and Functions Explained
- The Golgi Apparatus: Structure, Functions, and Cellular Importance
- Cytoplasm and Nucleus: Structure, Functions, Differences, and Examples
- What Is Cell Signaling? Structure, Types, and Functions Explained
- Three Main Parts of a Nucleotide: Structure and Functions
- Enzymes: Definition, Structure, Classification, and Functions
- DNA: Structure, Function, Discovery, and Modern Applications
- DNA Replication: Steps, Process, and Genetic Importance
- Properties of DNA: Structure, Physical, and Chemical Characteristics
- DNA Packaging: How DNA Fits into the Nucleus of a Cell
- R-Factors and Plasmids: How Bacteria Develop Antibiotic Resistance
- Chromosomes: Structure, Types, Functions, and Role in Genetics
- Genetic Material: DNA, RNA, and Their Role in Heredity and Evolution
- Deoxyribose and Ribose: Structure and Function in DNA and RNA
- Differences Between Genes and DNA: Definitions, Structures, and Functions
- RNA: Definition, Structure, Types, Functions, and Its Role in Life
Frequently Asked Questions
FAQ 1: What is DNA packaging, and why is it important?
DNA packaging is the process of neatly folding and organizing the long DNA molecule to fit inside a cell’s tiny nucleus or nucleoid while keeping it accessible for functions like replication and gene expression. This is a critical biological process that ensures cells can manage their genetic material efficiently.
- Space Efficiency: Human DNA is about 2 meters long per cell, but the nucleus is only a few micrometers wide. Packaging compacts DNA up to 10,000-fold to fit this space.
- Protection: By wrapping DNA around proteins like histones, packaging shields it from damage by enzymes or physical stress.
- Gene Regulation: Packaging determines which genes are active (in loosely packed euchromatin) or silent (in tightly packed heterochromatin), controlling cell functions like growth or immune response.
- Replication and Division: Organized DNA ensures accurate copying and distribution during cell division, preventing errors that could lead to diseases like cancer.
- Example: In sperm cells, DNA is ultra-compacted with protamines to streamline genetic delivery during fertilization, showcasing packaging’s role in reproduction.
This process is vital for all organisms, from bacteria to humans, as it balances compactness with functionality.
FAQ 2: How does DNA packaging differ between prokaryotes and eukaryotes?
DNA packaging varies significantly between prokaryotes (like bacteria) and eukaryotes (like plants and animals) due to differences in cell structure and genome size.
- Location: Prokaryotes lack a nucleus, so DNA is packaged in a region called the nucleoid within the cytoplasm. Eukaryotes package DNA in a membrane-bound nucleus.
- Proteins Involved: Eukaryotes use histones to form nucleosomes, while prokaryotes rely on nucleoid-associated proteins (NAPs) like HU and FIS for compaction.
- DNA Shape: Prokaryotic DNA is usually circular and supercoiled, whereas eukaryotic DNA is linear and organized into chromosomes.
- Compaction Level: Eukaryotes achieve higher compaction (up to 10,000-fold) due to larger genomes, while prokaryotes compact smaller genomes (e.g., E. coli’s 4.6 million base pairs) less extensively.
- Regulation: Eukaryotic packaging is tightly linked to epigenetic modifications, controlling gene expression. Prokaryotic packaging focuses more on structural organization.
For instance, in bacteria, DNA supercoiling allows rapid access for replication, fitting their fast growth, while human cells use complex chromatin structures for precise gene control.
FAQ 3: What role do histones play in DNA packaging?
Histones are small, positively charged proteins that are essential for DNA packaging in eukaryotic cells, enabling compact storage and regulating gene activity.
- Nucleosome Formation: Histones (H2A, H2B, H3, H4) form an octamer, around which DNA wraps to create nucleosomes, the basic packaging unit, reducing DNA length by about 7-fold.
- Charge Neutralization: The negative charge of DNA‘s phosphate backbone is countered by histones‘ positive charge from lysine and arginine, allowing tight binding.
- Higher-Order Structure: Linker histone H1 stabilizes nucleosomes and promotes folding into a 30-nanometer fiber, further compacting DNA.
- Gene Regulation: Histone modifications like acetylation or methylation control whether DNA is accessible for transcription, influencing cell functions like differentiation.
- Example: In active genes, acetylation loosens histone-DNA interactions, allowing enzymes to access DNA, as seen in immune cells responding to infection.
Histones are not just structural; they are dynamic players in gene regulation, making them critical in development and disease.
FAQ 4: How does DNA packaging affect gene expression?
DNA packaging directly influences gene expression by controlling access to the genetic code, determining which genes are turned on or off in a cell.
- Euchromatin vs. Heterochromatin: Loosely packed euchromatin allows transcription machinery to access DNA, activating genes, while tightly packed heterochromatin silences them.
- Histone Modifications: Chemical changes like acetylation open up chromatin for transcription, while methylation (e.g., H3K27me3) compacts it, repressing genes.
- Dynamic Process: Packaging adjusts based on cell needs; for example, during development, stem cells loosen specific DNA regions to activate tissue-specific genes.
- Epigenetic Control: Modifications can be inherited across cell divisions, maintaining cell identity, like keeping skin cells distinct from nerve cells.
- Example: In cancer, abnormal histone methylation can silence tumor suppressor genes, leading to uncontrolled cell growth, highlighting packaging’s regulatory role.
By organizing DNA into accessible or inaccessible states, packaging acts like a switchboard for gene activity.
FAQ 5: What are the different levels of DNA packaging in eukaryotic cells?
DNA packaging in eukaryotes occurs in a hierarchical manner, progressively compacting the molecule into a functional form within the nucleus.
- Nucleosome (First Level): DNA wraps around histone octamers (H2A, H2B, H3, H4), forming nucleosomes, achieving about 7-fold compaction.
- 30-nm Fiber (Second Level): Nucleosomes coil into a solenoid or zigzag structure, often stabilized by H1 histones, compacting DNA another 6-fold.
- Looped Domains (Third Level): Fibers form loops attached to scaffold proteins like topoisomerase II, achieving up to 800-fold compaction and organizing genes into functional domains.
- Chromosomes (Fourth Level): During mitosis, loops condense into compact chromosomes, visible under a microscope, with total compaction up to 10,000-fold.
- Example: In human cells, the 3 billion base pairs of DNA are condensed into 46 chromosomes during cell division, ensuring accurate distribution to daughter cells.
Each level builds on the previous, balancing compactness with accessibility for cellular processes.
FAQ 6: What are histone modifications, and how do they impact DNA packaging?
Histone modifications are chemical changes to histone proteins that alter how tightly DNA is packaged, affecting gene expression and other cellular processes.
- Types of Modifications: Include acetylation, methylation, phosphorylation, ubiquitination, and newer ones like lactylation, each with specific effects.
- Effect on Chromatin: Acetylation reduces histone positive charge, loosening DNA for transcription. Methylation can tighten (e.g., H3K9me) or loosen (e.g., H3K4me) chromatin.
- Enzymes Involved: Histone acetyltransferases (HATs) add acetyl groups, while histone deacetylases (HDACs) remove them. Histone methyltransferases (HMTs) add methyl groups.
- Biological Roles: Modifications regulate transcription, DNA repair, and cell division. For instance, phosphorylation on H3S10 aids chromosome condensation during mitosis.
- Example: In brain development, H3K27me3 represses non-neural genes in neurons, ensuring cell specialization, while its disruption can lead to disorders like Rett syndrome.
These modifications form a histone code that fine-tunes DNA accessibility, linking packaging to cellular function.
FAQ 7: Why is DNA packaging essential for cell division?
DNA packaging is crucial during cell division to ensure accurate replication and distribution of genetic material to daughter cells.
- Compaction for Segregation: During mitosis, DNA condenses into chromosomes, making it easier to separate evenly into new cells without tangling.
- Protection During Division: Tight packaging protects DNA from physical stress and enzymatic damage during chromosome movement.
- Regulation of Replication: Packaging organizes DNA into domains, ensuring replication starts at specific points, preventing errors.
- Histone Role: Histones and their modifications, like phosphorylation, signal condensation, while cohesins hold sister chromatids together until separation.
- Example: Errors in packaging during mitosis can lead to aneuploidy, where cells have incorrect chromosome numbers, as seen in Down syndrome (extra chromosome 21).
Proper packaging ensures genetic fidelity, critical for healthy cell division and organism development.
FAQ 8: How does DNA packaging in prokaryotes work without histones?
Prokaryotes, like bacteria, package their DNA in the nucleoid without histones, using alternative mechanisms to achieve compaction.
- Supercoiling: DNA is twisted into tight coils by enzymes like topoisomerases and gyrases, reducing length significantly.
- Nucleoid-Associated Proteins (NAPs): Proteins like HU, IHF, and FIS bend, loop, or wrap DNA, organizing it into compact domains.
- Circular DNA: Most prokaryotic DNA is circular, which naturally aids in compaction compared to linear eukaryotic DNA.
- Dynamic Access: Supercoiling and NAPs allow quick unwinding for replication and transcription, suiting fast-growing bacteria.
- Example: In E. coli, the 4.6 million base pair genome is compacted into a 1-micrometer nucleoid, enabling rapid division every 20 minutes under ideal conditions.
This simpler system is highly efficient for smaller genomes and rapid cellular processes.
FAQ 9: How does DNA packaging relate to diseases like cancer?
Faulty DNA packaging can disrupt gene regulation, leading to diseases like cancer by altering how genes are turned on or off.
- Histone Modification Errors: Abnormal acetylation or methylation can silence tumor suppressor genes or activate oncogenes, promoting uncontrolled cell growth.
- Chromatin Structure Changes: Disrupted nucleosome positioning or chromatin looping can expose DNA to damage or misregulate genes.
- Epigenetic Therapies: Drugs like HDAC inhibitors are used in cancer treatment to restore normal chromatin states, reactivating silenced genes.
- Specific Examples: In leukemia, H3K27me3 hypermethylation silences protective genes, while in breast cancer, altered acetylation patterns drive tumor growth.
- DNA Repair Impact: Packaging errors can impair DNA repair, leading to mutations that accumulate in cancerous cells.
Understanding packaging defects is key to developing targeted therapies for cancer and other disorders.
FAQ 10: What are the latest discoveries in DNA packaging research?
Recent advances in DNA packaging research have deepened our understanding of its role in biology and medicine, uncovering new mechanisms and applications.
- Novel Histone Modifications: Discoveries like lactylation and crotonylation link DNA packaging to metabolism, influencing gene expression in diseases like diabetes.
- 3D Genome Organization: Techniques like Hi-C reveal how chromatin loops create topologically associating domains (TADs), regulating long-range gene interactions.
- Single-Cell Studies: New methods allow scientists to study packaging in individual cells, showing how it varies in development or disease states like Alzheimer’s.
- Therapeutic Potential: Targeting packaging enzymes (e.g., HATs or HMTs) is being explored for cancer, neurodegenerative diseases, and epigenetic disorders.
- Example: Research on lactylation in tumor microenvironments suggests it promotes cancer growth, offering a potential target for immunotherapy.
These findings highlight DNA packaging as a dynamic field with far-reaching implications for health and disease.
FAQ 11: How has our understanding of DNA packaging evolved over time?
The journey to understanding DNA packaging began in the late 19th century, long before the double helix was unveiled. In 1869, Swiss chemist Friedrich Miescher isolated a substance he called “nuclein” from white blood cells, which we now know as DNA. At the time, he noted its acidic nature and association with proteins in the nucleus, but its role in heredity and packaging was unclear. This discovery laid the groundwork, though it took decades for scientists to connect it to genetic material. By the early 20th century, researchers like Phoebus Levene identified nucleotides as DNA’s building blocks, but the focus remained on chemistry rather than structure or compaction.
The breakthrough came in 1953 when James Watson and Francis Crick proposed the double-helix model, highlighting DNA’s twisted ladder shape and anti-parallel strands. This model explained replication but raised questions about fitting such a long molecule into cells. In the 1970s, Roger Kornberg described the nucleosome, showing how DNA wraps around histone proteins like beads on a string, achieving initial compaction. This was a pivotal moment, revealing packaging’s role in gene regulation.
Advancements in the 1980s and 1990s, using X-ray crystallography and electron microscopy, uncovered higher-order structures like the 30-nm fiber and chromatin loops. The Human Genome Project in the early 2000s shifted focus to epigenetics, showing how packaging influences gene expression without altering DNA sequence. Recent studies, using cryo-electron microscopy, have visualized dynamic packaging in real-time, linking it to diseases and cell division. Today, our knowledge continues to evolve, integrating computational models to predict packaging effects on health and evolution.
FAQ 12: What are the key enzymes involved in histone modifications for DNA packaging?
Histone modifications are crucial for adjusting DNA packaging, and specific enzymes act as writers (adding groups) or erasers (removing them). These enzymes influence chromatin structure, affecting gene access and cellular processes.
| Enzyme Type | Examples | Function | Impact on DNA Packaging | Biological Relevance |
|---|---|---|---|---|
| Histone Acetyltransferases (HATs) | p300/CBP, GCN5 | Add acetyl groups to lysine residues, reducing histone positive charge | Loosens chromatin, promoting euchromatin formation | Enhances transcription; dysregulation linked to cancer |
| Histone Deacetylases (HDACs) | HDAC1, HDAC4, SIRT1 | Remove acetyl groups, increasing histone-DNA affinity | Tightens chromatin into heterochromatin | Represses genes; inhibitors used in cancer therapy |
| Histone Methyltransferases (HMTs) | EZH2, SETD2, DOT1L | Add methyl groups to lysine or arginine | Can activate (e.g., H3K4me) or repress (e.g., H3K27me3) genes | Regulates development; mutations cause neurological disorders |
| Histone Demethylases (HDMs) | LSD1, JMJD family | Remove methyl groups | Reverses methylation effects, allowing dynamic regulation | Involved in stem cell differentiation and aging |
| Histone Kinases | ATM, ATR | Add phosphate groups, often in response to DNA damage | Alters chromatin for repair or condensation during mitosis | Critical for genome stability; defects accelerate aging |
| Histone Ubiquitinases | RNF20, BMI1 | Add ubiquitin, marking for degradation or signaling | Modulates nucleosome stability and gene silencing | Links to DNA repair; altered in cancers like leukemia |
These enzymes maintain a balance in histone states, ensuring proper packaging and response to cellular needs.
FAQ 13: What is DNA packaging like in viruses?
DNA packaging in viruses is a precise process where genetic material is compacted into a protein shell called a capsid, enabling efficient infection and replication.
- Genome Compaction: Viral DNA, often double-stranded, is packed at high density using motor proteins that generate force to overcome electrostatic repulsion, achieving densities similar to crystalline states.
- Packaging Motors: ATP-driven motors, like those in bacteriophages, use portal structures to thread DNA into the capsid, transitioning between cyclic and helical modes for efficient insertion.
- Ionic Influences: Salts and ions modulate packaging by neutralizing charges, affecting the speed and stability of DNA insertion into the capsid.
- Ejection Mechanism: Once packaged, DNA is rapidly ejected during infection, driven by internal pressure, to deliver the genome into host cells.
- Variations in Giant Viruses: Some large viruses use sheath-like structures or condensing proteins for even denser packing, adapting to their complex genomes.
This mechanism ensures viruses can hijack host machinery while protecting their DNA during transmission.
FAQ 14: How does DNA packaging influence evolution?
DNA packaging has profoundly shaped evolution by enabling complex genomes and precise gene regulation. In early life forms, simple supercoiling in prokaryotes allowed compact storage of circular DNA, facilitating rapid replication in bacteria. As eukaryotes emerged, the adoption of histones from archaea marked a key transition, wrapping linear DNA into nucleosomes and permitting larger genomes without tangling. This compaction, up to 10,000-fold, supported the expansion of genetic material, driving diversification from single-celled organisms to multicellular life.
Packaging also influences mutation rates and gene expression, acting as an evolutionary filter. Tightly packed heterochromatin protects DNA from damage but can silence genes, while loose euchromatin promotes variation through recombination. In gene transfer agents, virus-like particles derived from packaging machinery facilitate horizontal gene transfer, accelerating adaptation in microbes. During eukaryogenesis, a regime shift in genome size occurred, with chromatin structuralization enabling atmospheric oxygenation and the rise of plants and animals.
Moreover, epigenetic changes in packaging can be inherited, influencing traits across generations without altering the sequence. This “soft inheritance” has implications for rapid evolution in changing environments, such as in response to stressors. Overall, DNA packaging isn’t just a storage solution; it’s a dynamic force that has propelled life’s complexity over billions of years.
FAQ 15: What are the differences in DNA packaging between plants and animals?
While both plants and animals use similar DNA packaging mechanisms in eukaryotes, differences arise from evolutionary adaptations, genome sizes, and cellular needs.
| Aspect | Plants | Animals | Key Implications |
|---|---|---|---|
| Histone Variants | More divergent linker histones; stronger conservation in globular domains | Less divergence; animal-specific variants like H2A.Z | Plants adapt to environmental stress; animals focus on rapid development |
| Chromatin Organization | Lack CTCF-like insulators; more repetitive DNA in heterochromatin | Use CTCF for looping and insulation | Plants rely on RNA-directed methylation; animals have precise gene boundaries |
| Genome Size and Compaction | Larger genomes with polyploidy; extensive transposons | Smaller, more stable genomes | Plants tolerate duplications for adaptation; animals emphasize efficiency |
| Methylation Patterns | Methylation in all contexts (CG, CHG, CHH); dynamic in response to stress | Primarily CG methylation | Plants use it for silencing transposons; animals for gene imprinting |
| Nucleoid in Organelles | Mitochondrial nucleoids differ, with unique DNA-binding proteins | Similar but with animal-specific packaging | Reflects endosymbiotic origins; affects energy production |
These variations highlight how packaging supports plant resilience versus animal mobility.
FAQ 16: What laboratory techniques are used to study DNA packaging?
Scientists employ various techniques to investigate DNA packaging, combining biochemistry, imaging, and genomics for detailed insights.
- Chromatin Immunoprecipitation (ChIP): Binds antibodies to histones or proteins, isolating associated DNA to map packaging sites and modifications.
- Cryo-Electron Microscopy (Cryo-EM): Freezes samples to visualize nucleosomes and chromatin fibers at high resolution, revealing dynamic structures.
- DNase I Hypersensitivity Assays: Digests accessible chromatin regions, identifying loose packaging in active genes.
- Hi-C Sequencing: Captures 3D interactions, mapping how DNA loops and folds within the nucleus.
- Reconstituted Systems: Uses egg extracts or synthetic nucleosomes to simulate packaging, studying real-time compaction.
These methods help link packaging to function and disease.
FAQ 17: What are the future directions in DNA packaging research?
Research into DNA packaging is poised for exciting advancements, focusing on its links to health, technology, and fundamental biology. Scientists are exploring how packaging regulates replication speed, with recent findings showing it directly influences cell division rates. This could lead to therapies targeting packaging errors in cancer, where abnormal chromatin hinders tumor suppressors like p53. Future studies may use AI to predict packaging dynamics, integrating 3D genomics for personalized medicine.
Another frontier is DNA as a storage medium, leveraging its density for archiving vast data sustainably. Innovations in tagging and encapsulation could revolutionize computing, with medical applications in cold data storage for genomes. Epigenetic editing, building on CRISPR, aims to manipulate packaging without sequence changes, potentially reversing age-related defects.
Additionally, real-time imaging of sperm DNA folding offers insights into fertility, while mobile forensic tools analyze packaging for crime-solving. As climate change intensifies, understanding environmental impacts on packaging will be crucial. Overall, this field promises breakthroughs in treating diseases, enhancing data tech, and unraveling life’s complexities.
FAQ 18: How do environmental factors affect DNA packaging?
Environmental influences can alter DNA packaging through epigenetic changes, impacting gene expression and health.
| Factor | Description | Effect on Packaging | Health Implications | Examples |
|---|---|---|---|---|
| Temperature and Humidity | Heat or moisture accelerates DNA degradation | Disrupts histone binding, loosening chromatin | Increases mutation risk; affects fertility | High temperatures cause unpackaging in sperm |
| Chemical Pollutants | Toxins like heavy metals or pesticides | Alters methylation, making DNA more accessible | Raises cancer and inflammation risks | Arsenic exposure leads to hypermethylation |
| Diet and Nutrition | Nutrient deficiencies (e.g., folate) | Changes acetylation patterns | Influences development and aging | High-fat diets promote repressive modifications |
| Stress and Hormones | Chronic stress elevates cortisol | Phosphorylates histones, compacting regions | Linked to mental health disorders | Stress silences growth-related genes |
| UV Radiation and Light | Exposure to sunlight or artificial light | Damages DNA, prompting repair remodeling | Accelerates skin aging and cancer | UV causes demethylation in exposed cells |
These factors highlight packaging’s responsiveness to surroundings.
FAQ 19: How does DNA packaging function in stem cells?
In stem cells, DNA packaging maintains pluripotency and guides differentiation, balancing openness for potential with control for specialization.
- Chromatin Plasticity: Loose packaging in pluripotent regions allows access to genes like Oct4, enabling self-renewal.
- Nucleosome Dynamics: High-resolution mapping shows unique folding in embryonic stem cells, with increased domains in cancer stem cells.
- Epigenetic Regulation: Modifications like H3K27me3 repress lineage genes, while activation opens cardiac pathways.
- Aging and Renewal: Packaging changes reduce renewal capacity, linking to regenerative decline.
- Disease Links: In Down syndrome, extra chromosomes alter packaging, raising leukemia risk in stem-like cells.
This flexibility is key to stem cell versatility.
FAQ 20: What is the connection between DNA packaging and aging?
DNA packaging plays a central role in aging, as gradual disruptions in chromatin structure contribute to cellular decline. Over time, histones may misalign, leading to improper gene expression and increased vulnerability to damage. For instance, in older cells, heterochromatin erodes, exposing DNA to stressors and promoting inflammation. This unpacking can activate dormant viruses or silence repair genes, accelerating senescence.
Epigenetic clocks, based on methylation patterns, measure biological age by tracking packaging changes. Accumulated DNA damage from environmental factors overwhelms repair systems, causing breaks that remodel chromatin. In fertility, aging oocytes show packaging defects, reducing viability. Telomere shortening, part of packaging at chromosome ends, signals cell death when critical.
Interventions targeting packaging, like enhancing histone stability, could mitigate aging effects. By understanding these links, research aims to extend healthy lifespan, viewing aging as a malleable process influenced by how our genome is stored.
Acknowledgement
The educational website Examsmeta.com expresses its gratitude to the scientific community and various reputable sources that have significantly contributed to the creation of the article “DNA Packaging: How DNA Fits into the Nucleus of a Cell.” The comprehensive insights provided by Nature, NCBI, and Cell have been instrumental in shaping the detailed and accurate content of this article. Their rigorous research and accessible publications have enriched our understanding of DNA packaging, from its historical context to modern advancements.
Below are the key contributions from these sources:
- Nature: Provided foundational studies on the double-helix structure and recent discoveries in epigenetic modifications, offering a robust historical and contemporary perspective.
- NCBI: Offered detailed reviews and primary research articles on histone modifications, chromatin organization, and prokaryotic DNA packaging, ensuring scientific accuracy.
- Cell: Contributed cutting-edge insights into 3D genome organization and the role of DNA packaging in disease, enhancing the article’s depth and relevance.

