Mitochondria are often called the powerhouses of the cell, but they’re far more than just energy factories. These organelles are constantly changing shape, splitting apart and merging together in a process known as mitochondrial dynamics. This ongoing dance helps cells adapt to their needs, from producing ATP to managing stress and even deciding when it’s time for a cell to die. At the heart of this process is dynamin-related protein 1 (DRP1), a protein that acts like a molecular scissor, cutting mitochondria into smaller pieces through fission. When everything works smoothly, DRP1 keeps things balanced. But if it goes into overdrive or slows down too much, it can lead to serious health issues, from brain disorders to heart problems and even cancer.
Imagine your mitochondria as a network of tubes in a busy city. Sometimes, you need to break them apart to reroute traffic or isolate a damaged section. That’s where DRP1 comes in. It’s a GTPase enzyme, meaning it uses energy from GTP molecules to do its job. Without DRP1, cells struggle to divide their mitochondria properly during growth or to remove faulty ones, leading to a buildup of problems. Researchers have found that tweaking DRP1 could be a game-changer for treating various diseases, but it’s not straightforward because its effects depend on the context, like the type of cell or the stage of illness.
Table of Contents
In everyday life, mitochondrial fission helps distribute these organelles evenly during cell division, ensuring new cells get their fair share. It also plays a role in quality control, spotting and isolating damaged parts so they can be recycled. But when DRP1 is disrupted, the whole system falters. For instance, in nerve cells that need a lot of energy, too much fission can fragment mitochondria, making it hard to transport energy along long axons. This is why DRP1 is linked to conditions where energy supply is critical.
The Intricate Mechanism Behind DRP1-Mediated Mitochondrial Fission
DRP1 doesn’t work alone; it’s part of a complex system. Normally, it hangs out in the cell’s cytoplasm, forming small groups like dimers or tetramers, waiting for a signal. When the cell needs to split a mitochondrion, DRP1 gets recruited to the outer mitochondrial membrane by adapter proteins. These include Mitochondrial Fission Factor (Mff), MiD49, MiD51, and sometimes Fis1. These adapters act like docking stations, pulling DRP1 right where it’s needed, often at spots where the mitochondrion touches the endoplasmic reticulum.
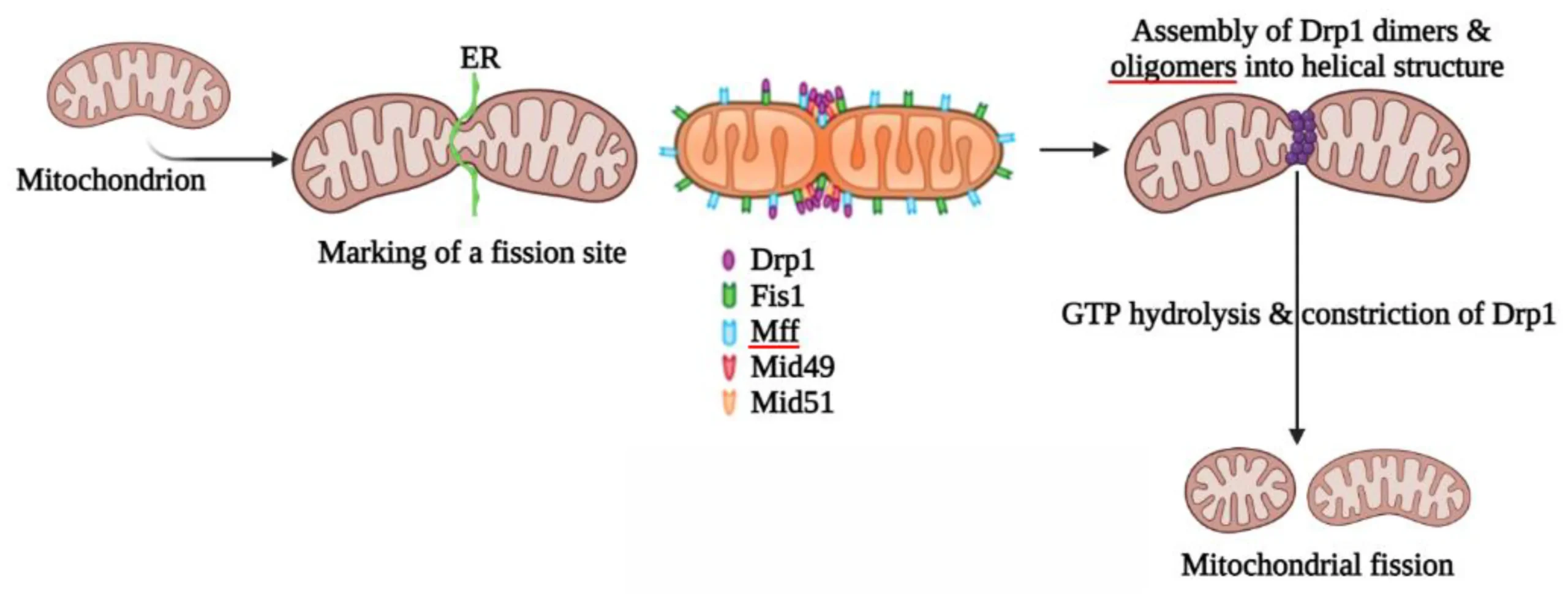
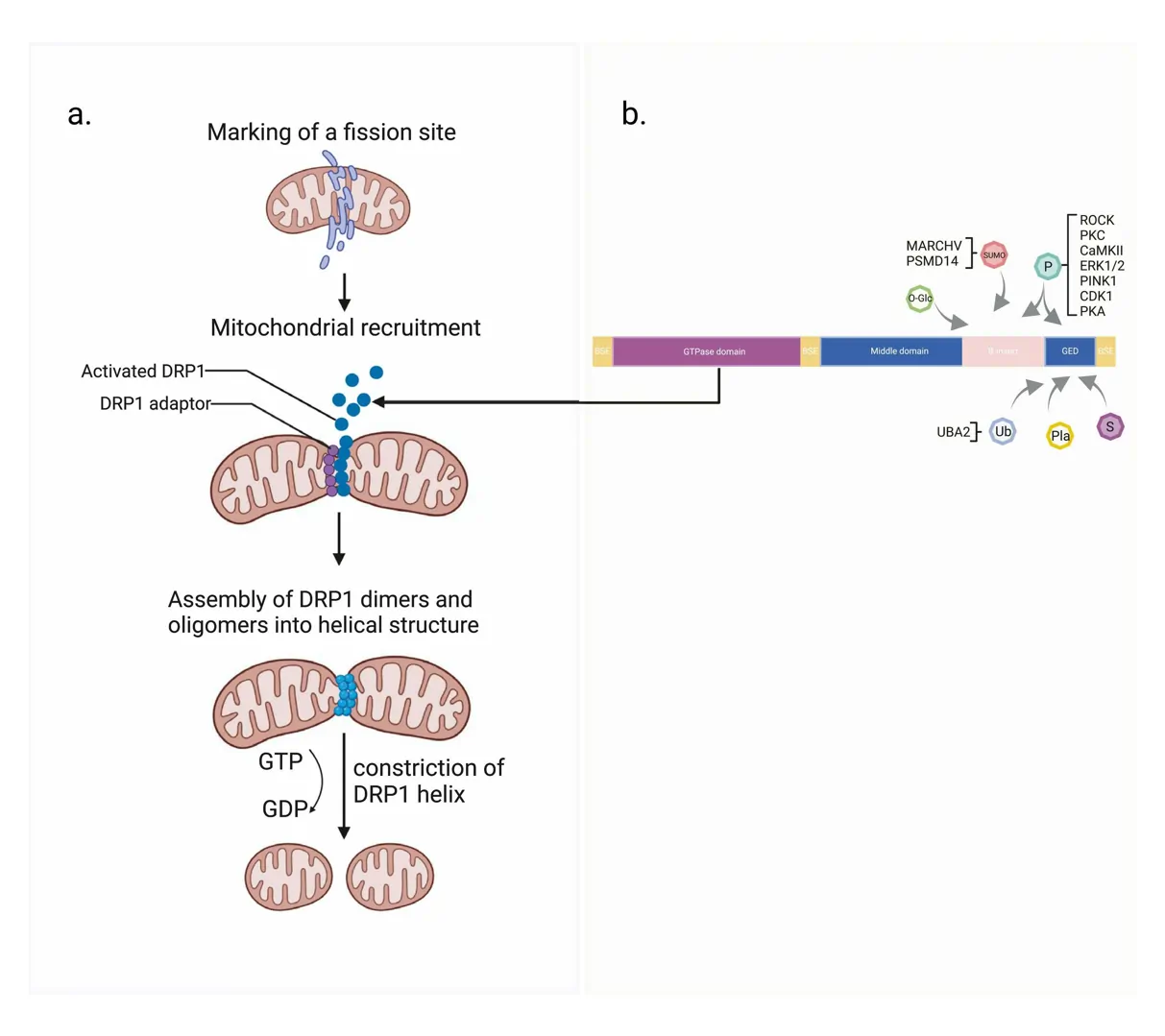
Once there, DRP1 starts assembling into a spiral ring around the mitochondrion, much like a coil tightening around a hose. This ring constricts the organelle, powered by the hydrolysis of GTP. The reaction can be represented as $$ \text{DRP1-GTP} \rightarrow \text{DRP1-GDP} + \text{P}_i $$, where the energy released drives the shape change. This mechanical force squeezes the membranes until they snap apart, creating two separate mitochondria. But it’s not just about cutting; the endoplasmic reticulum and actin filaments help pre-constrict the site, making DRP1’s job easier. Cardiolipin, a special lipid in the mitochondrial membrane, also helps DRP1 stick and assemble properly.
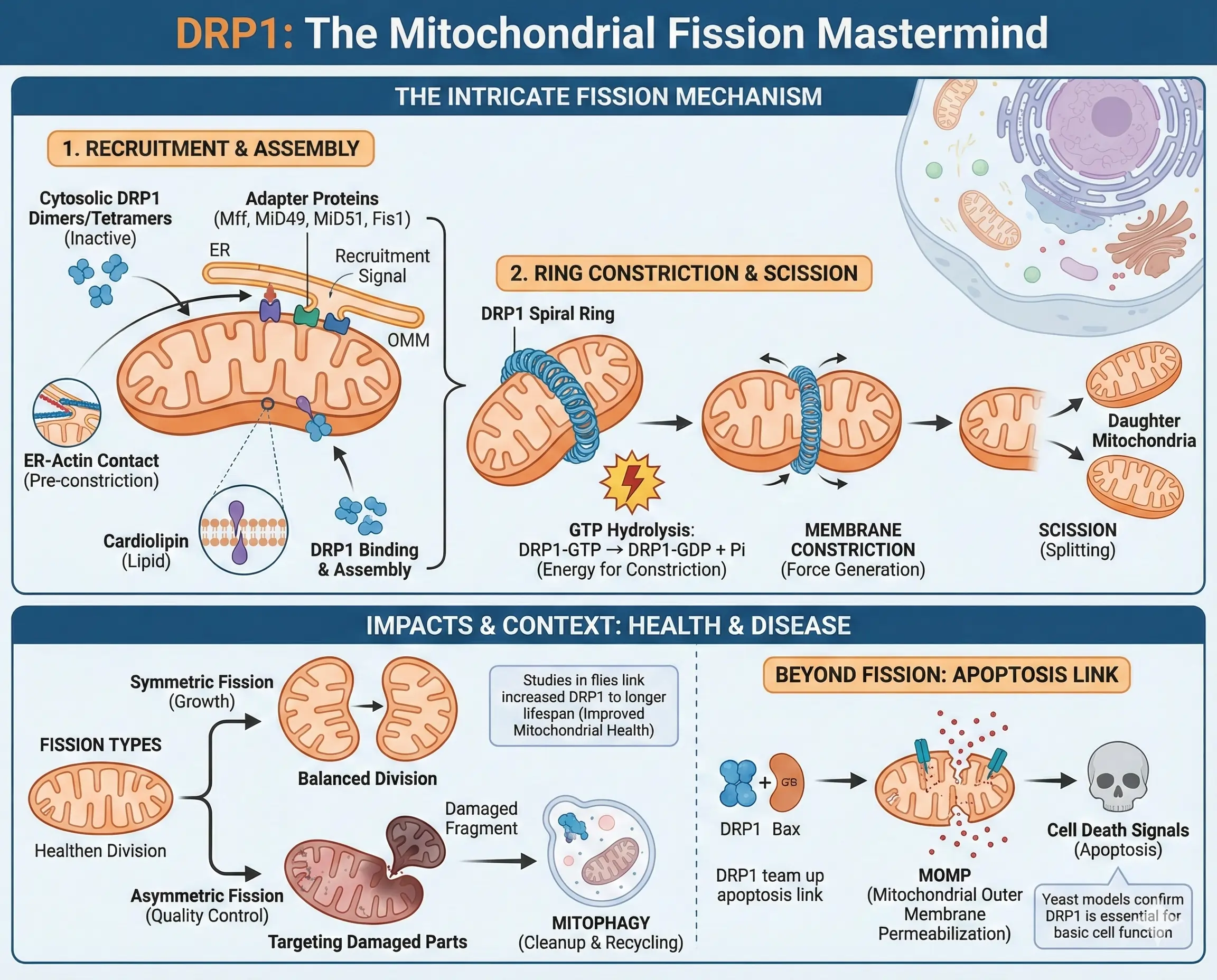
Think of it like baking bread. You knead the dough (pre-constriction by ER and actin), then slice it (DRP1’s ring tightens), and finally separate the loaves (scission). If any step fails, you end up with uneven or stuck-together pieces. In yeast cells, similar proteins show how essential this is for basic cell function, and studies in flies have even linked increased DRP1 activity to longer lifespans by improving mitochondrial health.
Fission isn’t always the same. There’s symmetric fission for growth, where healthy mitochondria divide evenly, and asymmetric fission for cleanup, targeting damaged parts with low membrane potential. This damaged bit then gets tagged for mitophagy, where lysosomes break it down. DRP1’s role here connects to bigger processes like apoptosis, where it teams up with proteins like Bax to release signals that trigger cell death.
How DRP1 Activity is Finely Tuned Through Regulation
DRP1’s power is kept in check by a web of controls, mainly through post-translational modifications that flip switches on its behavior. These changes happen after the protein is made and can activate, inhibit, or even mark it for destruction.
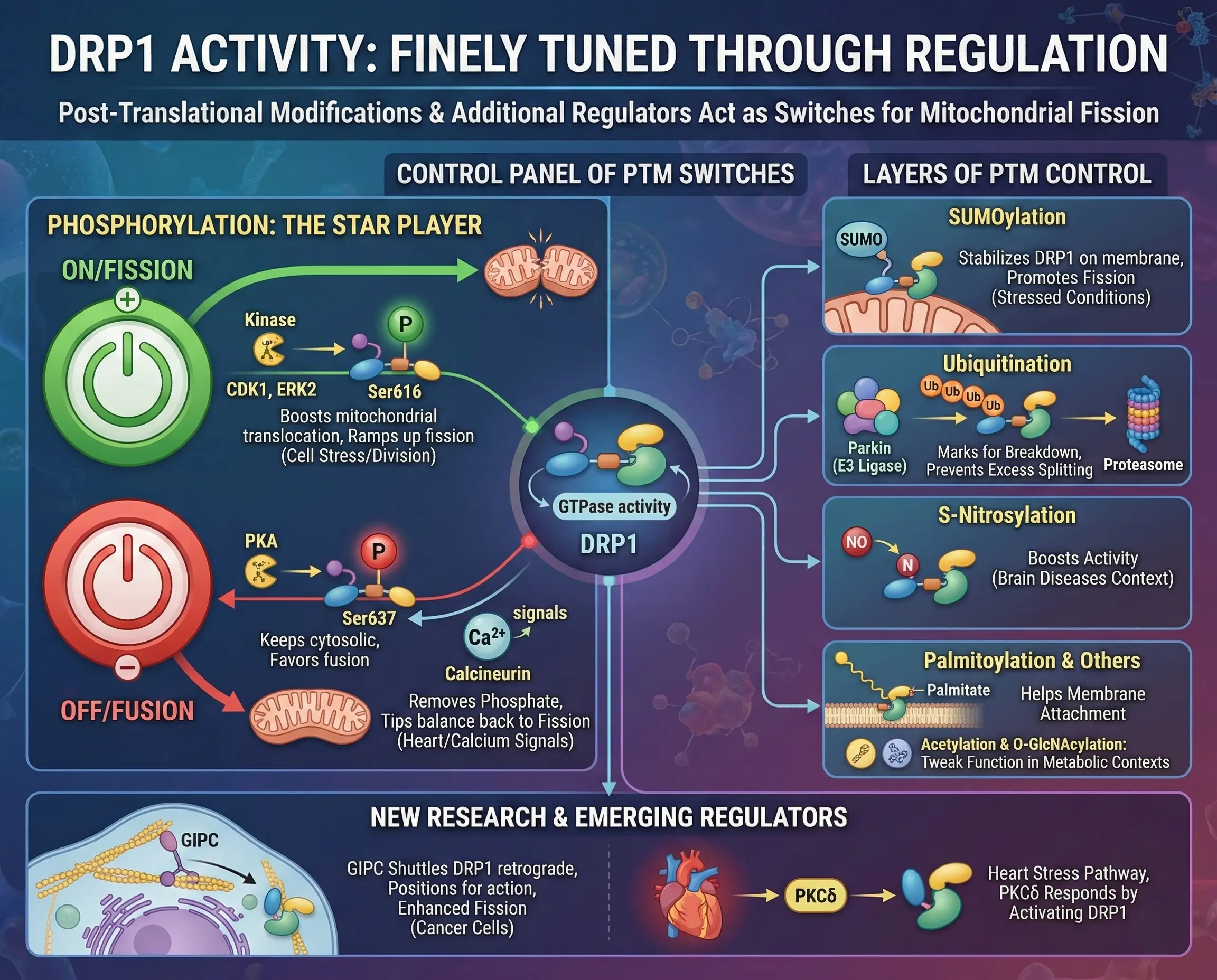
Phosphorylation is the star player. Adding a phosphate group at specific spots can rev up or slow down DRP1. For example, phosphorylation at Serine 616 by kinases like CDK1 or ERK2 boosts its move to mitochondria, ramping up fission during cell stress or division. On the flip side, phosphorylation at Serine 637 by PKA keeps it stuck in the cytoplasm, favoring fusion instead. Calcineurin, a phosphatase, can remove this phosphate, tipping the balance back to fission, especially in heart cells under calcium signals.
Other modifications add layers of control. SUMOylation attaches SUMO proteins to DRP1, stabilizing it on the membrane for more fission, often seen in stressed conditions. Ubiquitination, handled by enzymes like Parkin, can lead to DRP1 breakdown, preventing too much splitting. S-Nitrosylation boosts its activity in brain diseases, while palmitoylation helps with membrane attachment. Even acetylation and O-GlcNAcylation tweak its function in metabolic contexts.
New research points to additional regulators. For instance, GIPC protein helps shuttle DRP1 back toward the cell’s center along actin tracks, enhancing fission in cancer cells. This retrograde transport doesn’t change DRP1’s enzyme activity but positions it better for action. In heart tissue, pathways involving PKCδ respond to stress by activating DRP1.
| Post-Translational Modification | Key Sites | Regulating Enzymes | Effect on DRP1 Activity | Examples in Context |
|---|---|---|---|---|
| Phosphorylation | Ser616 | CDK1, ERK2, GSK3β | Activation, promotes translocation and fission | Increased in cancer proliferation and Alzheimer’s fragmentation |
| Phosphorylation | Ser637 | PKA | Inhibition, retains in cytoplasm, favors fusion | Dephosphorylated by calcineurin in cardiac ischemia |
| SUMOylation | Lys residues (e.g., K594) | MAPL ligase | Stabilization on membrane, enhances fission | Involved in bone cell regulation and apoptosis |
| Ubiquitination | Various Lys | PINK1, Parkin | Degradation, reduces excessive fission | Linked to Parkinson’s mitophagy defects |
| S-Nitrosylation | Cys644 | Nitric oxide sources | Enhances GTPase and dimerization | Elevated in neurodegenerative diseases like Huntington’s |
| Palmitoylation | Cys residues | DHHC enzymes | Membrane association | Helps in fission during metabolic stress |
| Acetylation | Lys residues | Acetyltransferases | Modulates activity in energy metabolism | Altered in diabetes and obesity |
| O-GlcNAcylation | Ser/Thr residues | OGT enzyme | Influences phosphorylation crosstalk | Affects fission in high-glucose environments |
This table shows how diverse these modifications are, each responding to different signals like stress, nutrients, or hormones. In flies, upregulating DRP1 extends lifespan, suggesting balanced regulation could promote healthy aging.
DRP1’s Dual Role: From Cellular Hero to Disease Villain
DRP1 is like a double-edged sword. In healthy cells, it maintains balance, but when dysregulated, it fuels disease. In the brain, excessive DRP1 causes mitochondrial fragments that clog axons, leading to synaptic failure. In Alzheimer’s, amyloid-beta proteins bind DRP1, boosting fission and contributing to memory loss. Similar patterns appear in Parkinson’s, where faulty mitophagy leaves damaged mitochondria piling up.
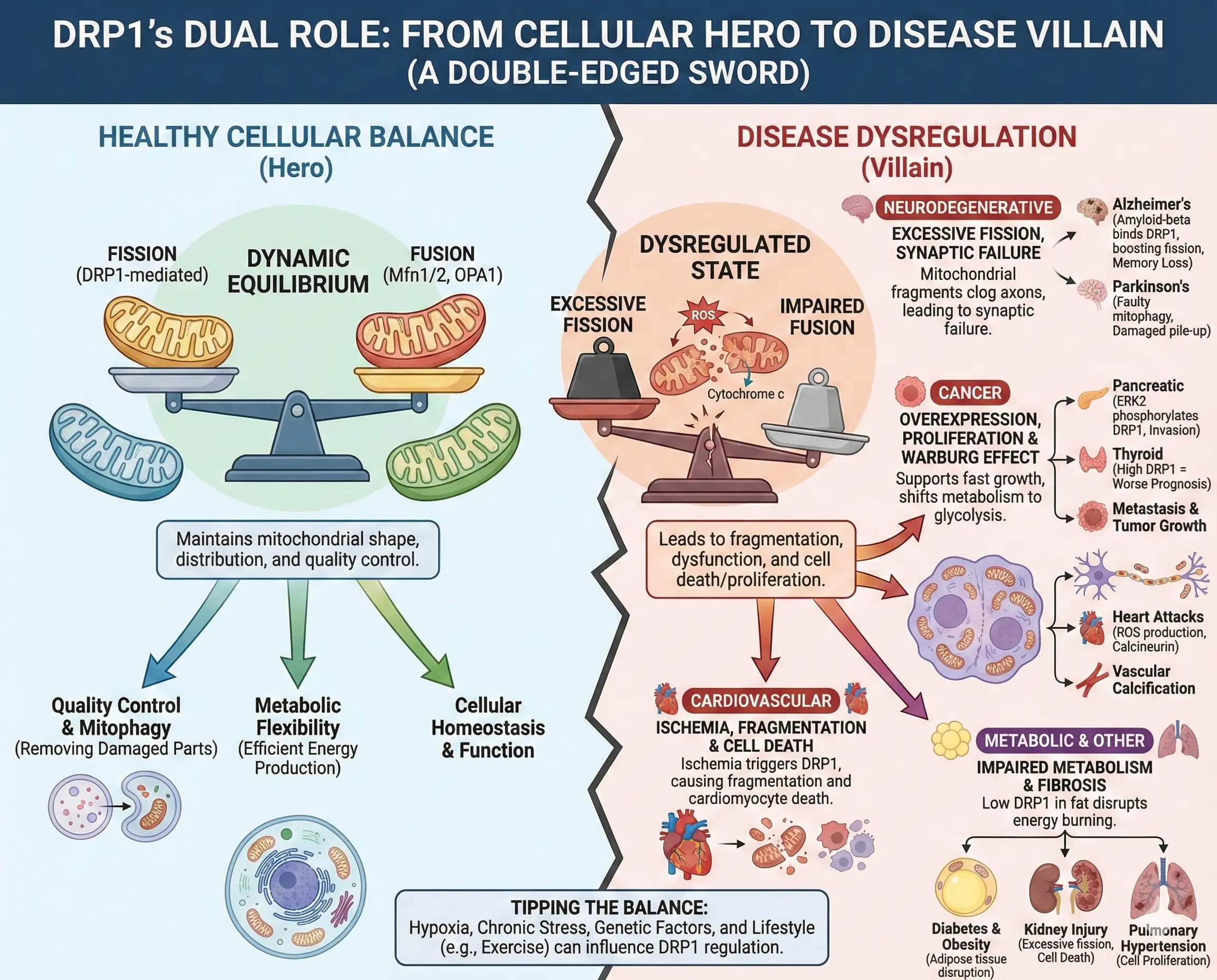
Cancer is trickier. Many tumors crank up DRP1 to support fast growth and shift metabolism to glycolysis, the Warburg effect. In pancreatic cancer, ERK2 phosphorylates DRP1, driving invasion. But in some cases, too much fission stresses cells, slowing tumor spread. High DRP1 often means worse prognosis, like in thyroid or brain cancers.
Heart diseases highlight DRP1’s role in energy-demanding tissues. During heart attacks, ischemia triggers DRP1, causing fragmentation and cell death. Inhibiting it protects against this. In diabetes, low DRP1 in fat tissue disrupts energy burning, worsening obesity.
Even in lungs and kidneys, DRP1 matters. In pulmonary hypertension, it promotes cell proliferation, while in kidney injury, excessive fission leads to cell death. Exercise might help by moderating DRP1, reducing chronic disease risks.
| Disease Category | Role of DRP1 Dysregulation | Key Mechanisms | Potential Outcomes | Examples from Studies |
|---|---|---|---|---|
| Neurodegenerative (e.g., Alzheimer’s, Parkinson’s) | Excessive fission and fragmentation | Interaction with amyloid-beta, tau; S-nitrosylation | Synaptic dysfunction, neuronal death | Elevated DRP1 in AD brains across stages; Mdivi-1 reduces ischemia damage |
| Cancer (e.g., Pancreatic, Thyroid) | Overexpression promotes proliferation | ERK2 phosphorylation at Ser616; metabolic shift | Tumor growth, metastasis, poor survival | Ras signaling enhances fission in PDAC; Mdivi-1 slows oncocytic cell growth |
| Cardiovascular (e.g., Heart Failure, Ischemia) | Increased fission during stress | Calcineurin dephosphorylation; ROS production | Cardiomyocyte apoptosis, calcification | DRP1 inhibition attenuates vascular calcification in models |
| Metabolic (e.g., Diabetes, Obesity) | Reduced or altered expression | High-fat diet effects; acetylation changes | Impaired energy metabolism | Disrupted morphology in adipose tissue |
| Other (e.g., Renal, Pulmonary) | Excessive fission in injury | Hypoxia/ischemia activation | Cell death, fibrosis | Linked to sepsis and hypertension progression |
These examples show DRP1’s broad impact. In hypoxia, like during strokes, DRP1’s oligomer changes maintain quality but can go awry.
Therapeutic Horizons: Targeting DRP1 for Better Health
With DRP1’s fingerprints on so many diseases, it’s a hot target for drugs. Inhibitors like Mdivi-1 block its GTPase activity, reducing fission and protecting cells in heart attack models. But Mdivi-1 has side effects, hitting other targets like mitochondrial complex I. Newer options like P110, a peptide that stops DRP1 from binding Fis1, show promise without those issues. P110 reduces pathology in brain injuries, sepsis, and neurodegeneration, and might help in diabetes.
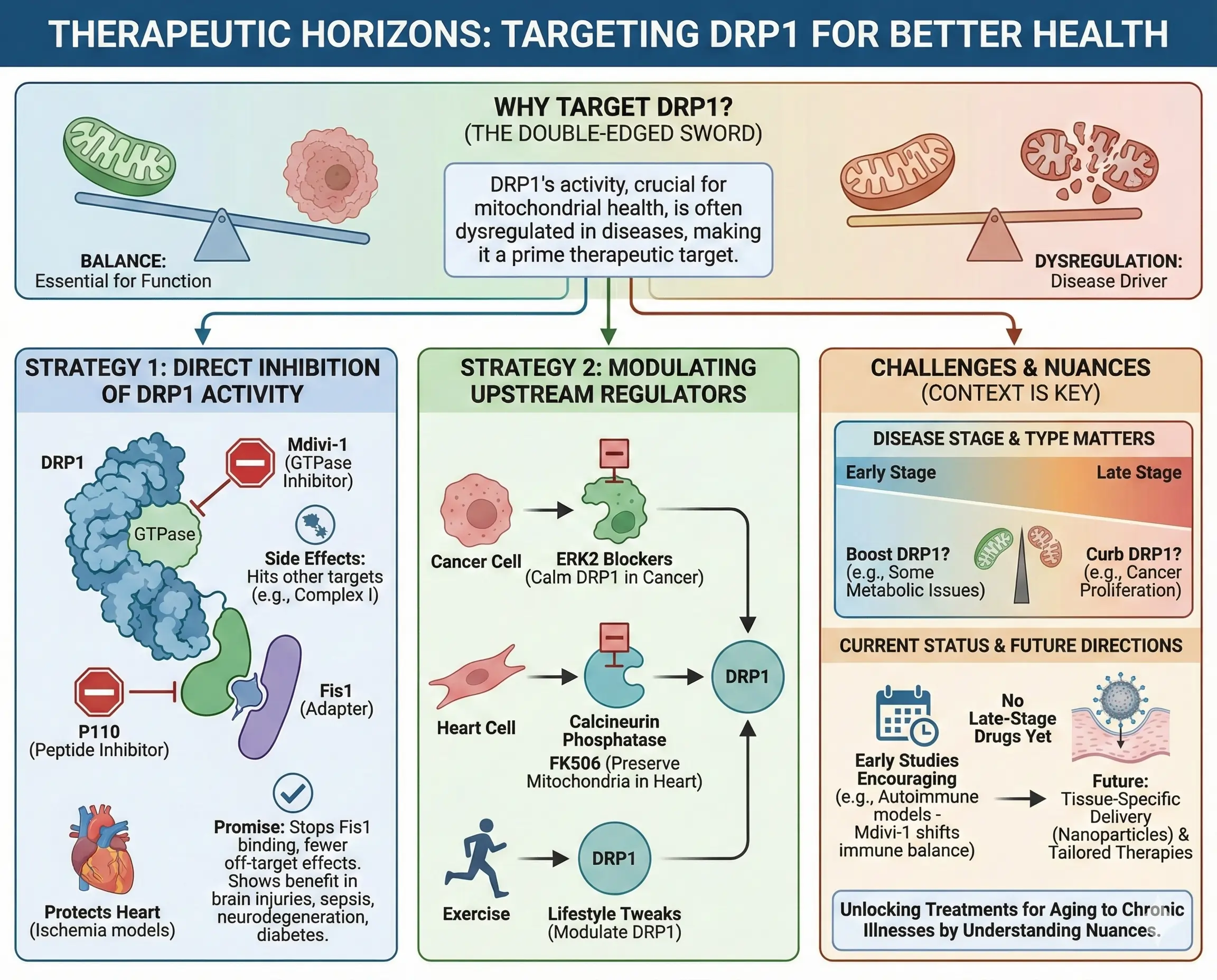
Other approaches target upstream regulators. For cancer, blocking ERK2 could calm DRP1. In hearts, calcineurin inhibitors like FK506 preserve mitochondria. Even exercise modulates DRP1, suggesting lifestyle tweaks alongside drugs.
Challenges remain. DRP1’s effects vary by disease stage—one might need to boost it in some metabolic issues but curb it in cancer. No DRP1 drugs are in late-stage trials yet, but early studies are encouraging. For instance, in autoimmune models, Mdivi-1 shifts immune cell balance, hinting at broader uses.
Future research might tailor therapies, like using nanoparticles to deliver inhibitors to specific tissues. By understanding DRP1’s nuances, we could unlock treatments for everything from aging to chronic illnesses.
In wrapping up, DRP1 isn’t just a protein—it’s a gatekeeper of cellular fate. Balancing its activity could revolutionize medicine, turning insights into real-world relief. As science digs deeper, expect more breakthroughs in this fascinating field.
Frequently Asked Questions
FAQ 1: What Exactly Is DRP1 and How Does It Control Mitochondrial Fission in Cells?
Dynamin-related protein 1, commonly known as DRP1, is a crucial protein that acts like a molecular machine in our cells, specifically handling the division of mitochondria. These tiny organelles are responsible for generating energy, and they need to split and merge to stay healthy and functional. Without proper fission, which is the splitting process, mitochondria can become too large or damaged, leading to cellular problems. DRP1 belongs to the dynamin family of GTPases, meaning it uses GTP as fuel to power its actions. In a resting state, it floats around in the cell’s cytoplasm, forming small clusters like dimers or tetramers, ready to spring into action when needed.
The process starts when signals in the cell recruit DRP1 to the outer membrane of the mitochondria. Adapter proteins such as Mitochondrial Fission Factor (Mff), MiD49, MiD51, and sometimes Fis1 guide it to the right spots, often where the mitochondrion contacts the endoplasmic reticulum. Here, actin filaments help create an initial squeeze. Once attached, DRP1 assembles into a spiral ring around the mitochondrion, tightening like a noose as it hydrolyzes GTP. This mechanical constriction leads to the actual division, separating one mitochondrion into two. The inner membrane follows suit, ensuring complete fission. Recent structural studies from 2024 have shown how DRP1’s hinge regions allow flexible conformations, enabling this helical assembly on lipid membranes like cardiolipin-rich areas.
This fission isn’t random; it serves different purposes based on the cell’s needs. In growing cells, symmetric fission ensures even distribution during division. In stressed or damaged mitochondria, asymmetric fission isolates faulty parts for recycling through mitophagy. Disruptions in this can link to diseases, but in healthy scenarios, it maintains energy production and cellular balance. Advances in understanding DRP1’s interactions with lipids like cardiolipin highlight how it activates only at specific sites, preventing unnecessary divisions and supporting overall mitochondrial health.
FAQ 2: How Do Post-Translational Modifications Regulate DRP1 Function?
Post-translational modifications are like chemical tags added to DRP1 after it’s made, fine-tuning its behavior in response to cellular signals. These changes dictate whether DRP1 promotes fission or stays inactive, impacting everything from energy metabolism to cell survival. For instance, phosphorylation at certain sites can activate or inhibit it, while other modifications like SUMOylation stabilize it on the membrane.
| Modification Type | Key Sites Involved | Enzymes Responsible | Impact on DRP1 | Disease Relevance |
|---|---|---|---|---|
| Phosphorylation | Serine 616 | CDK1, ERK2, GSK3β | Activates translocation to mitochondria, boosts fission | Elevated in cancer and Alzheimer’s, contributing to fragmentation and cell stress |
| Phosphorylation | Serine 637 | PKA, PKCδ | Inhibits activity, keeps DRP1 in cytoplasm, promotes fusion | Dephosphorylation by calcineurin in heart ischemia leads to excessive fission |
| SUMOylation | Lysine residues (e.g., K594) | MAPL ligase, SENP3 deSUMOylase | Stabilizes on membrane, enhances fission | Involved in apoptosis and bone cell regulation; dysregulation in neurodegeneration |
| Ubiquitination | Various lysine sites | PINK1, Parkin, MARCH5 | Leads to degradation, reduces excessive fission | Defective in Parkinson’s, linked to mitophagy failure |
| S-Nitrosylation | Cysteine 644 | Nitric oxide sources | Increases GTPase activity and dimerization | Heightened in Huntington’s and Alzheimer’s, promoting toxic fragmentation |
| ISGylation | Lysine sites | HERC5 ligase | Balances other modifications, regulates dynamics | Newly identified in 2024, fine-tunes fission in immune responses and stress |
| Palmitoylation | Cysteine residues | DHHC enzymes | Aids membrane association | Supports fission during metabolic shifts in diabetes |
| Acetylation | Lysine residues | Acetyltransferases | Modulates in energy metabolism | Altered in obesity, affecting mitochondrial morphology |
| O-GlcNAcylation | Serine/Threonine sites | OGT enzyme | Influences phosphorylation crosstalk | Impacts fission in high-glucose environments like type 2 diabetes |
These modifications create a dynamic regulatory network, ensuring DRP1 responds precisely to needs like stress or growth. Recent 2025 research emphasizes how ISGylation counterbalances ubiquitination, offering new therapeutic angles for balancing mitochondrial dynamics.
FAQ 3: What Role Does DRP1 Play in Neurodegenerative Diseases Such as Alzheimer’s and Parkinson’s?
DRP1 has emerged as a central figure in neurodegenerative diseases, where its overactivity often leads to harmful mitochondrial fragmentation. In Alzheimer’s disease, proteins like amyloid-beta and phosphorylated tau directly interact with DRP1, ramping up its fission activity. This causes mitochondria to break into small, inefficient pieces, disrupting energy supply in neurons and impairing synaptic function. Over time, this fragmentation contributes to neuronal death and cognitive decline, as seen in brain imaging studies showing shrunken mitochondria in affected areas.
In Parkinson’s disease, DRP1’s dysregulation ties into faulty mitophagy, where damaged mitochondria aren’t properly cleared. Mutations in genes like Parkin reduce DRP1 ubiquitination, leading to unchecked fission and accumulation of toxic organelles. This exacerbates oxidative stress and dopamine neuron loss, core to the disease’s motor symptoms.
- Excessive DRP1-driven fission in Huntington’s links to S-nitrosylation, promoting mutant huntingtin aggregation and neuronal vulnerability.
- In epileptic encephalopathies, patient mutations in DRP1 impair synaptic maturation, causing developmental delays and seizures, as revealed in 2024 studies using cortical neuron models.
- Partial DRP1 knockout in mice improves autophagy flux, suggesting therapeutic potential for reducing protein aggregates in these disorders.
Overall, targeting DRP1 could mitigate these effects, with inhibitors showing promise in reducing inflammation and mitochondrial dysfunction in Alzheimer’s models.
FAQ 4: How Does DRP1 Contribute to Cancer Progression and What Makes It a Complex Target?
In many cancers, DRP1 is overexpressed, fueling rapid cell growth by reshaping mitochondrial networks to support high energy demands. This shift enables the Warburg effect, where cells prefer glycolysis over efficient oxidative phosphorylation, allowing quick proliferation even in low-oxygen environments. For example, in pancreatic and thyroid cancers, ERK2 phosphorylates DRP1 at Serine 616, enhancing fission and promoting invasion and metastasis.
However, DRP1’s role isn’t always pro-cancer; in some contexts, excessive fission induces metabolic stress that curbs tumor growth. High DRP1 levels often correlate with poor prognosis, as seen in gliomas where it remodels mitochondrial cristae to boost oxidative phosphorylation, driving aggressive progression. Targeting the DRP1-FIS1 axis in high-grade gliomas inhibits this remodeling, slowing tumor advance.
The complexity arises from context-dependency: in oncocytic tumors, inhibiting DRP1 with Mdivi-1 slows growth, but in others, it might have opposite effects. Recent 2025 research highlights DRP1’s involvement in cardio-oncology, where it mediates doxorubicin-induced cardiotoxicity while aiding cancer cell survival, underscoring the need for precise, tumor-specific therapies.
FAQ 5: What Are the Key Implications of DRP1 in Cardiovascular Diseases?
DRP1’s influence on heart health is profound, given the organ’s high energy needs. Excessive fission triggered by stress like ischemia can lead to cell death and failure.
| Cardiovascular Condition | DRP1’s Role | Mechanisms Involved | Potential Outcomes | Therapeutic Insights |
|---|---|---|---|---|
| Heart Failure | Increased fission | Calcineurin dephosphorylates Ser637, promoting fragmentation | Cardiomyocyte apoptosis, reduced contractility | DRP1 inhibition attenuates damage in models |
| Ischemia/Reperfusion Injury | Hyperactivation | ROS and calcium signals boost DRP1 translocation | Mitochondrial swelling, energy crisis | Mdivi-1 protects against reperfusion harm |
| Vascular Calcification | Excessive activity | PKCδ pathway activates fission | Plaque buildup, stiffness | Inhibitors reduce calcification progression |
| Doxorubicin Cardiotoxicity | Upregulated fission | Interacts with FIS1, disrupts dynamics | Cardiomyocyte death in cancer patients | P110 peptide shows promise in cardio-oncology |
| Pulmonary Hypertension | Promotes proliferation | Hypoxia activates DRP1 | Vessel remodeling, right heart strain | Targeting reduces cell overgrowth |
Balancing DRP1 through exercise or drugs could prevent these issues, as 2025 studies link reduced DRP1 to better outcomes in chronic heart conditions.
FAQ 6: Are There Effective Therapeutic Strategies Targeting DRP1 for Various Diseases?
Developing therapies around DRP1 is promising but challenging due to its dual roles. Inhibitors aim to curb excessive fission in diseases like neurodegeneration and heart conditions.
- Mdivi-1: The most studied small molecule, it blocks DRP1’s GTPase activity, protecting against ischemia and Alzheimer’s in models, though off-target effects on complex I limit clinical use.
- P110 Peptide: Targets DRP1-Fis1 interaction, effective in sepsis, brain injury, and doxorubicin cardiotoxicity without broad side effects.
- Upstream Modulators: Drugs like TDZD-8 inhibit GSK3β to prevent Ser616 phosphorylation, reducing fission in neurotoxicity.
- Lifestyle Interventions: Exercise lowers DRP1 activity, combating chronic diseases like diabetes by improving mitochondrial health.
Future directions include isoform-specific targeting, as different DRP1 variants affect diseases variably, and nanoparticle delivery for precision.
FAQ 7: How Does DRP1 Interact with Other Proteins to Maintain Mitochondrial Dynamics?
DRP1 doesn’t operate in isolation; it collaborates with a suite of proteins to orchestrate fission. Adapter proteins on the outer membrane recruit it, while interactions with the endoplasmic reticulum and cytoskeleton set the stage. For instance, Mff and MiD proteins anchor DRP1, forming fission sites often at ER-mitochondria contact points where INF2 and actin pre-constrict.
Once assembled, DRP1’s helical structure interacts with cardiolipin lipids, enhancing GTPase activity for constriction. In fusion-fission balance, DRP1 opposes proteins like OPA1 and MFN1/2. Dysregulation, as in MARCH5-mediated ubiquitination, affects assembly and disassembly.
New 2024 cryo-EM studies reveal DRP1’s lattice on membranes, showing dimer conformations that drive efficient fission, offering insights into how mutations disrupt these interactions in encephalopathies.
FAQ 8: What Recent Advances Have Been Made in DRP1 Research as of 2025?
Recent DRP1 research has uncovered exciting links to metabolism, apoptosis, and targeted therapies.
| Advance Area | Key Findings | Year and Details | Implications |
|---|---|---|---|
| Structural Insights | Flexible hinge 1 allows extended/contracted conformations in dimers | 2024, cryo-EM studies | Better understanding of assembly for drug design |
| Apoptosis Connection | DRP1 ties mitochondrial fission to metabolism and cell death pathways | 2025 review | Potential for anti-cancer and anti-degenerative drugs |
| Cardio-Oncology | DRP1 mediates doxorubicin toxicity; inhibitors protect heart | 2024-2025 studies | Dual benefit in cancer treatment without heart damage |
| Patient Mutations | De novo DRP1 mutations impair synaptic maturation in neurons | 2024 exome sequencing | Explains epileptic encephalopathies, guides gene therapies |
| Autophagy Flux | Partial knockout enhances autophagy independent of mitochondria | 2024 mouse models | Benefits for Parkinson’s and metal toxicity |
| Glioma Progression | DRP1-FIS1 axis remodels cristae for OXPHOS in high-grade tumors | 2024 machine learning analysis | New target for impeding aggressive cancers |
These breakthroughs pave the way for personalized medicine.
FAQ 9: How Does DRP1 Affect Metabolic Disorders Like Obesity and Type 2 Diabetes?
In metabolic disorders, DRP1‘s dysregulation disrupts mitochondrial morphology, impairing energy handling. High-fat diets often decrease DRP1 expression in fat tissue, leading to elongated mitochondria that inefficiently burn fat, contributing to obesity and insulin resistance. This shift favors fat storage over expenditure, worsening systemic issues.
In type 2 diabetes, altered modifications like acetylation and O-GlcNAcylation in high-glucose settings promote unbalanced fission, causing oxidative stress and beta-cell dysfunction. Exercise counters this by moderating DRP1, enhancing fission-fusion balance for better glucose control. Recent findings link DRP1 to drug-induced toxicities, where inhibitors like Mdivi-1 ameliorate skeletal muscle wasting in metabolic stress.
FAQ 10: What Is the Connection Between DRP1 and Programmed Cell Death or Apoptosis?
DRP1 bridges mitochondrial fission and apoptosis, where excessive splitting releases death signals. During stress, DRP1 teams with Bax to fragment mitochondria, releasing cytochrome c to activate caspases and cell death.
This link is evident in diseases; in neurodegeneration, it amplifies toxic protein effects, while in cancer, it confers resistance by fine-tuning metabolism. 2025 reviews emphasize DRP1’s metabolic ties, making it a hub for apoptosis regulation.
- In hypoxia, oligomeric changes maintain quality but can trigger apoptosis if unchecked.
- SUMOylation enhances fission during apoptosis, stabilized by MAPL.
- Inhibitors block this in heart injury, preventing unnecessary cell loss.
FAQ 11: What Is the Role of DRP1 in Aging and Longevity?
DRP1, or dynamin-related protein 1, plays a fascinating and somewhat contradictory role in the aging process, influencing how our cells handle mitochondrial health over time. As we age, mitochondria tend to become less efficient, leading to increased oxidative stress, inflammation, and cellular decline. DRP1 steps in as the key regulator of mitochondrial fission, helping to break down these organelles into smaller units for quality control or distribution. However, excessive DRP1 activity can fragment mitochondria too much, contributing to age-related issues like reduced energy production and heightened inflammation. Studies show that in older tissues, such as the brain, DRP1 expression and phosphorylation increase, promoting more fission and impairing biogenesis—the creation of new mitochondria. This imbalance can accelerate cognitive decline and other hallmarks of aging, as fragmented mitochondria struggle to maintain the cell’s energy demands.
Interestingly, modulating DRP1 might hold the key to extending healthy lifespan. In model organisms like fruit flies, overexpressing DRP1 in midlife has been linked to longer lifespans and better health spans, possibly by enhancing mitophagy, the process where damaged mitochondria are cleared out. This cleanup helps prevent the buildup of dysfunctional organelles that fuel chronic inflammation and oxidative damage. On the flip side, reducing DRP1 during development in worms can extend lifespan in certain genetic backgrounds, like those with insulin signaling mutations, by improving stress resistance. These findings suggest DRP1’s effects are context-dependent—too much or too little can tip the scales toward pathology, but fine-tuning it could mimic the benefits of calorie restriction or other longevity interventions.
Recent 2025 research highlights how DRP1 intersects with pathways like the integrated stress response, where it influences metabolic adaptations during aging. For instance, in mice, protein restriction extends lifespan partly through DRP1 modulation, involving hormones like FGF21 that curb excessive fission. In human studies, elevated DRP1 in aged cerebral tissues correlates with impaired mitochondrial function, but interventions targeting DRP1, such as exercise or specific inhibitors, show promise in reversing these changes. By promoting a balanced mitochondrial network, these approaches could mitigate age-associated diseases, offering a pathway to healthier aging. Overall, DRP1 emerges as a double-edged sword in longevity: a potential villain in unchecked fission but a hero when regulated properly to support cellular resilience.
FAQ 12: How Does Exercise Influence DRP1 Activity and Mitochondrial Dynamics?
Exercise is a powerful modulator of mitochondrial health, and DRP1 sits at the center of how physical activity reshapes these organelles for better performance. Regular workouts, whether aerobic or resistance-based, can dial down excessive DRP1-driven fission, leading to more elongated mitochondria that enhance energy efficiency and reduce oxidative stress. This adaptation is crucial for muscles, where exercise reverses hyperactive fission seen in sedentary states or diseases like diabetes, improving respiratory capacity without necessarily boosting new mitochondrial creation.
| Exercise Type | Impact on DRP1 | Effects on Mitochondrial Dynamics | Benefits Observed | Supporting Evidence |
|---|---|---|---|---|
| Aerobic (e.g., Running, Cycling) | Reduces DRP1 phosphorylation at activating sites like Ser616 | Promotes fusion over fission, leading to elongated networks | Enhanced endurance, better fat oxidation, reduced fatigue | Exercise training reverses skeletal muscle DRP1 hyperactivation in diabetes models, improving capacity independent of biogenesis |
| Resistance Training | Suppresses DRP1 expression in stressed tissues | Balances fission-fusion, prevents fragmentation | Increased muscle strength, mitigated atrophy | Drp1 deficiency alters adaptations but exercise overcomes this for performance |
| High-Intensity Interval Training (HIIT) | Temporarily activates then normalizes DRP1 | Boosts mitophagy via controlled fission, remodels shape for oxidative boost | Improved metabolic flexibility, anti-inflammatory effects | Acute exercise identifies novel DRP1 role, enhancing dynamics in skeletal muscle |
| Endurance Exercise | Inhibits DRP1-mediated fragmentation in heart | Reduces fission during ischemia-reperfusion, preserves function | Cardioprotection, lower apoptosis risk | Training reduces fragmented mitochondria in cardiac models |
| Chronic Moderate Exercise | Downregulates DRP1 in aging tissues | Enhances overall network stability, increases biogenesis | Slows age-related decline, extends health span | Overexpression or silencing effects mimic exercise benefits in models |
| Acute Bout Exercise | Transient DRP1 activation for fission | Facilitates rapid adaptation, energy redistribution | Quick performance gains, reduced muscle damage | Regulates dynamics for physiology and mass maintenance |
These changes underscore exercise as a natural way to optimize DRP1, combating chronic conditions through mitochondrial remodeling.
FAQ 13: What Are the Key Structural Features of DRP1 and How Do They Function?
The structure of DRP1 is a marvel of molecular engineering, enabling it to act as a mechanochemical GTPase for organelle division. At its core, DRP1 consists of several domains that work together: the GTPase domain for energy harnessing, the middle domain for assembly, the GTPase effector domain (GED) for regulation, and a variable domain (VD) that interacts with lipids and adapters. Recent cryo-EM studies from 2025 reveal how DRP1 forms dimers in an auto-inhibited state, with a flexible hinge allowing transitions to active conformations for helical polymerization on membranes.
- GTPase Domain: This N-terminal region hydrolyzes GTP, providing the power for constriction. Mutations here, like G32A, impair hydrolysis, leading to diseases by disrupting fission.
- Middle Domain: Essential for oligomerization, it forms the stalk of the helical lattice, as seen in 2025 lattice structures on lipids.
- GED: Acts as a bundle signaling element, coordinating with the GTPase for conformational changes during disassembly post-hydrolysis.
- Variable Domain: Binds cardiolipin-rich membranes, enabling recruitment; its flexibility governs assembly.
These features allow DRP1 to constrict and sever, with hydrolysis $$ \text{DRP1-GTP} \rightarrow \text{DRP1-GDP} + \text{P_i} $$ driving the process. In pathologies, structural alterations from mutations highlight DRP1’s precision.
FAQ 14: How Does DRP1 Contribute to Immune Responses and Inflammation?
DRP1 bridges mitochondrial dynamics and immune function, where its fission activity can amplify inflammatory signals in response to threats like infections or stress. In immune cells such as macrophages, DRP1-mediated fission reorganizes mitochondria to boost energy for cytokine production, but excessive activity leads to chronic inflammation seen in diseases like atherosclerosis or neurodegeneration. For example, lipopolysaccharide from bacteria promotes DRP1-dependent fission, activating pathways like NF-κB and MAPK to release pro-inflammatory molecules such as TNF-α and IL-6. Inhibiting DRP1 curbs this, reducing inflammation without compromising basic defenses.
In viral infections, DRP1’s role flips somewhat; its depletion elongates mitochondria, enhancing anti-viral responses by clustering sensors like MAVS for stronger interferon signaling. This suggests DRP1 fine-tunes immunity—controlled fission aids acute responses, but unchecked it fuels pathology. In hepatocytes, DRP1 deficiency heightens inflammation, while in other contexts, it dampens it, showing cell-type specificity.
Recent insights link DRP1 to innate immunity via kinases like TBK1, which phosphorylates DRP1 to disable assemblies during RNA sensing, reprogramming metabolism for defense. In chronic settings, DRP1 knockdown attenuates cognitive deficits in aging by suppressing NLRP3 inflammasomes, highlighting its therapeutic potential in inflammatory disorders.
FAQ 15: What Novel Inhibitors of DRP1 Are Being Developed Beyond Mdivi-1?
Beyond the well-known Mdivi-1, researchers are exploring new DRP1 inhibitors with improved specificity and efficacy for treating fission-related diseases.
| Inhibitor Name | Mechanism of Action | Target Diseases/Applications | Advantages Over Mdivi-1 | Development Status |
|---|---|---|---|---|
| DRP1i1, DRP1i2, DRP1i3 | Disrupt protein-protein interactions at recruitment sites | Cardioprotection in ischemia | Specific to human DRP1, fewer off-targets | Preclinical, shows infarct reduction |
| Drpitor1a | GTPase inhibition, blocks oligomerization | Pulmonary hypertension, cancer | Higher potency, better pharmacokinetics | Effective in models, improvement over Mdivi-1 |
| P110 Peptide | Inhibits DRP1-FIS1 binding | Neurodegeneration, sepsis | No broad mitochondrial effects, targeted | Promising in multiple models |
| DRP1i27 | Allosteric disruption of assembly | Cancer, mitochondrial disorders | Hits specific conformations | Literature-reported, synergistic potential |
| NanoDRP1i1 | Nanoparticle-delivered DRP1i1 | Acute myocardial infarction | Cardiac-targeted delivery | Limits infarct size in vivo |
These inhibitors aim for precision, avoiding Mdivi-1’s side effects like complex I inhibition.
FAQ 16: What Is DRP1’s Role in Breast Cancer or Lung Cancer Progression?
In breast cancer, DRP1 often drives progression by enhancing mitochondrial fission, which supports rapid proliferation and metastasis. Overexpression leads to fragmented mitochondria, promoting metabolic shifts like increased glycolysis and OXPHOS for tumor demands. In triple-negative subtypes, enhanced fission suppresses signaling that would otherwise halt growth.
- Promoting migration: DRP1 repositions mitochondria for energy during invasion, with inhibition reducing metastasis in models.
- Stem cell maintenance: High DRP1 in cancer stem cells aids self-renewal, correlating with poor prognosis.
- Therapy resistance: Combining inhibitors with paclitaxel resensitizes cells.
In lung cancer, similar patterns emerge, with DRP1 fostering proliferation in hepatocellular types via fission, though specifics vary.
FAQ 17: What Genetic Mutations in DRP1 Are Associated with Human Diseases?
Mutations in DRP1 (DNM1L gene) disrupt fission, leading to severe neurological and multisystem disorders.
| Mutation | Domain Affected | Associated Diseases | Functional Impact | Clinical Features |
|---|---|---|---|---|
| G32A | GTPase | Encephalomyopathic form (EMPF1) | Impaired GTP hydrolysis, reduced assembly | Developmental delay, seizures, early lethality |
| A395D | GTPase | Lethal neonatal encephalopathy | Blocks higher-order assembly | Mitochondrial/peroxisomal defects, death |
| De novo variants (e.g., in middle domain) | Middle | Epileptic encephalopathies | Alters synaptic maturation | Cognitive deficits, epilepsy |
| R403C | GTPase | Optic atrophy plus | Perturbs dynamics | Vision loss, neurological issues |
These often cause elongated mitochondria and metabolic dysregulation.
FAQ 18: What Is the Role of DRP1 in Peroxisomal Fission?
DRP1 isn’t limited to mitochondria; it also drives peroxisomal fission, ensuring these organelles divide properly for lipid metabolism and ROS detoxification. Peroxisomes, like mitochondria, rely on DRP1’s GTPase activity to constrict and sever, with adapters like FIS1 and MFF recruiting it to their membranes. This shared machinery highlights evolutionary links, as defects in DRP1 affect both, leading to diseases with metabolic and neurological symptoms.
In healthy cells, DRP1 maintains peroxisomal numbers by fission, preventing over-elongation that impairs function. Mutations causing DRP1 loss result in elongated peroxisomes, disrupting fatty acid breakdown and causing accumulations toxic to nerves. Studies confirm DRP1’s dual constricting and severing roles, sufficient for both organelles without additional factors.
Recent work shows DRP1 adaptors like MiD49/51 work cooperatively for peroxisomal division, with deficiencies causing fission failure and pathologies like Zellweger spectrum disorders. This underscores DRP1’s broad cellular importance.
FAQ 19: How Evolutionarily Conserved Is DRP1 Across Species?
DRP1 is remarkably conserved, reflecting its essential role in eukaryotic organelle dynamics from yeast to humans. Homologs like Dnm1p in yeast perform similar fission, with core domains—GTPase, middle, GED—preserved for assembly and hydrolysis. This conservation allows insights from simple models to apply to complex diseases.
Interactions, such as with FIS1, are maintained across eukaryotes, enabling direct binding for recruitment. Post-translational sites, like Ser656 for PKA phosphorylation, are evolutionarily stable, regulating fission universally.
In flies, DRP1 ensures mitochondrial distribution during meiosis, mirroring yeast, while human mutations echo defects in lower organisms, aiding research.
FAQ 20: What Are the Future Directions in DRP1 Research as of 2025?
As of 2025, DRP1 research is expanding into precision medicine and novel applications.
- Targeted Therapies: Developing isoform-specific inhibitors for cancer, focusing on DRP1’s role in proliferation and resistance.
- Structural Insights: Using nanobodies for advanced imaging to track DRP1 in live cells.
- Aging Interventions: Exploring DRP1 modulation via diet or exercise to extend lifespan.
- Cardiovascular Focus: Investigating DRP1 in heart diseases for new drugs.
- Neurotoxicity Studies: Deeper dives into DRP1 in anesthetics like propofol.
- Metabolic Axes: Targeting SLC39A1-DRP1 in liver cancers for diagnostics.
- Apoptosis Links: Elucidating DRP1’s ties to metabolism for diagnostics.
Acknowledgments
The creation of the article “DRP1: How Dynamin-Related Protein 1 Shapes Mitochondrial Dynamics, Health, and Disease” was made possible through the wealth of scientific knowledge available from several reputable sources. The Examsmeta.com expresses its gratitude to the following platforms for their comprehensive and reliable information, which significantly enriched the content and depth of this article:
- Nature (www.nature.com) for its extensive collection of peer-reviewed studies on mitochondrial dynamics and DRP1’s role in cellular processes.
- ScienceDirect (www.sciencedirect.com) for providing detailed research articles on DRP1’s structural biology and its implications in diseases like cancer and neurodegeneration.
- PubMed (pubmed.ncbi.nlm.nih.gov) for its vast database of biomedical literature, offering critical insights into DRP1’s post-translational modifications and therapeutic potential.
- Cell (www.cell.com) for cutting-edge publications on DRP1’s interactions with other proteins and its evolutionary conservation across species.
- Journal of Clinical Investigation (www.jci.org) for in-depth studies on DRP1’s role in metabolic disorders and cardiovascular diseases, aiding the therapeutic perspective of the article.

