Juxtacrine signaling represents one of the most intimate forms of communication between cells in living organisms. Unlike signals that travel through fluids or over long distances, this process requires cells to be in direct physical contact, allowing them to exchange information precisely and efficiently. Imagine two neighbors chatting over a fence instead of shouting across a street; that’s the essence of juxtacrine signaling. It’s crucial for everything from building tissues during development to mounting an effective immune defense.
In this comprehensive article, we’ll dive deep into what it is, how it works, its various types, real-world examples, and its broader implications in health, disease, and even unicellular life. We’ll explore insights from biological research to paint a full picture, making complex ideas accessible and engaging.
Table of Contents
What Is Juxtacrine Signaling?
Juxtacrine signaling is a type of cell-to-cell communication that demands close proximity. Cells don’t release signaling molecules into the surrounding space like in other methods; instead, a ligand on one cell’s surface binds directly to a receptor on an adjacent cell’s surface. This contact-dependent approach ensures signals are targeted and localized, preventing unnecessary widespread effects. It’s particularly vital in multicellular organisms where precise coordination is key, but it also appears in simpler forms of life like bacteria.
Think about how cells in your body form organs or respond to threats. Without juxtacrine signaling, these processes could go awry, leading to developmental issues or weakened immunity. Researchers have observed this signaling in growth factors, cytokines, and chemokines, which are proteins that help regulate cell behavior.
For instance, it plays a starring role in the immune response by allowing immune cells to activate each other upon contact. It’s also central to cell fate determination, where one cell influences another’s destiny, such as becoming a neuron or a muscle cell.
In contrast to diffusive signaling, where molecules float freely, or transport via nanotubes and vesicles, juxtacrine signaling avoids dilution and ensures high specificity. This makes it ideal for fine-tuned control in crowded cellular environments, like during embryo formation or wound healing.
The Three Main Types of Juxtacrine Signaling
Biologists classify juxtacrine signaling into three primary categories, each highlighting a different way cells interact directly. These types underscore the versatility of this signaling method, from membrane interactions to matrix involvement.
First, there’s the interaction between a membrane-bound ligand and a membrane protein on adjacent cells. Here, proteins, lipids, or oligosaccharides on one cell’s surface latch onto receptors on the neighboring cell, triggering a response. This is like a handshake that conveys specific instructions.
Second, communicating junctions link the intracellular compartments of two cells, permitting the passage of small molecules. These junctions act as tiny tunnels, allowing ions and signaling molecules to flow directly from one cytoplasm to another.
Third, an extracellular matrix glycoprotein interacts with a membrane protein. The matrix, a supportive scaffold around cells, provides ligands that bind to cell receptors, influencing behavior like migration or differentiation.
These types aren’t mutually exclusive; they often overlap in complex biological scenarios, enhancing the robustness of cellular communication.
Type 1: Membrane-Bound Ligand and Receptor Interactions
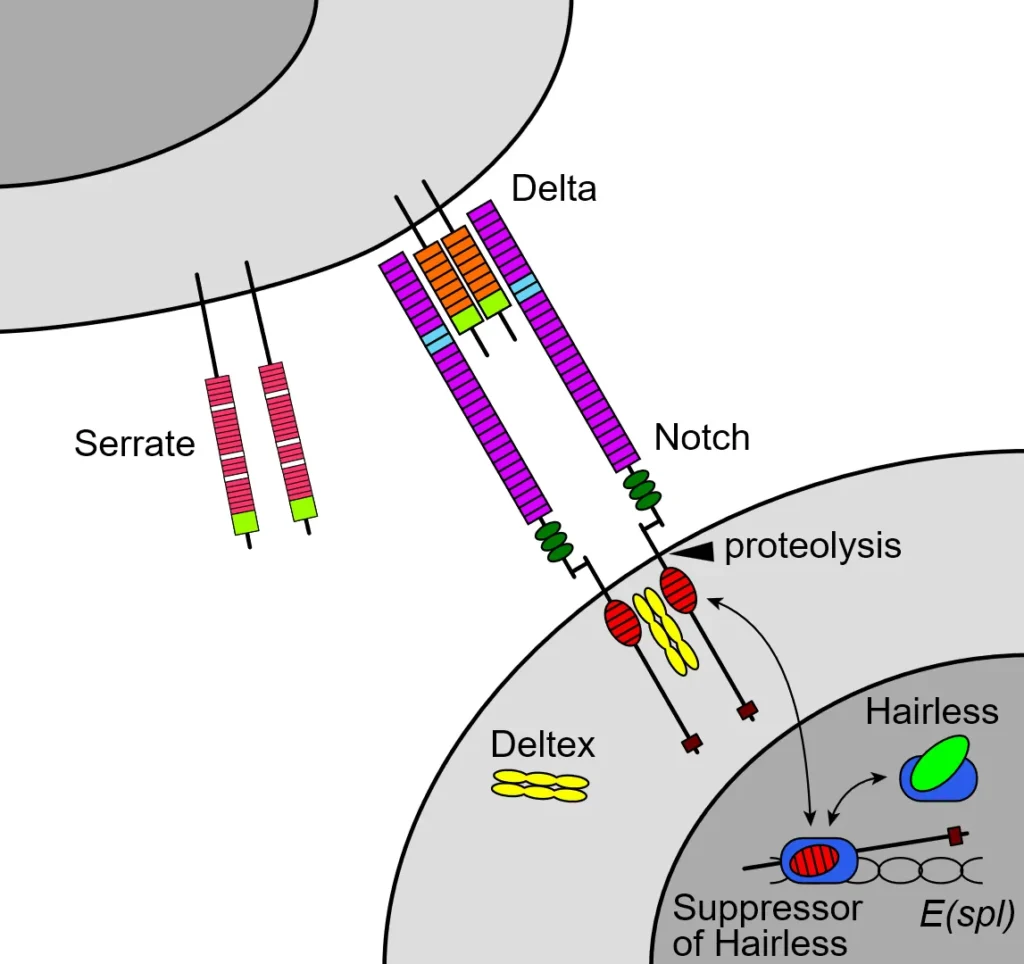
In this type, the signaling molecule stays anchored to the sending cell’s membrane, ensuring the signal only affects touching cells. A classic example is the Notch pathway, where the Delta ligand on one cell binds to the Notch receptor on another. This binding causes the Notch receptor to cleave, releasing a fragment that enters the nucleus and alters gene expression, often deciding cell fates.
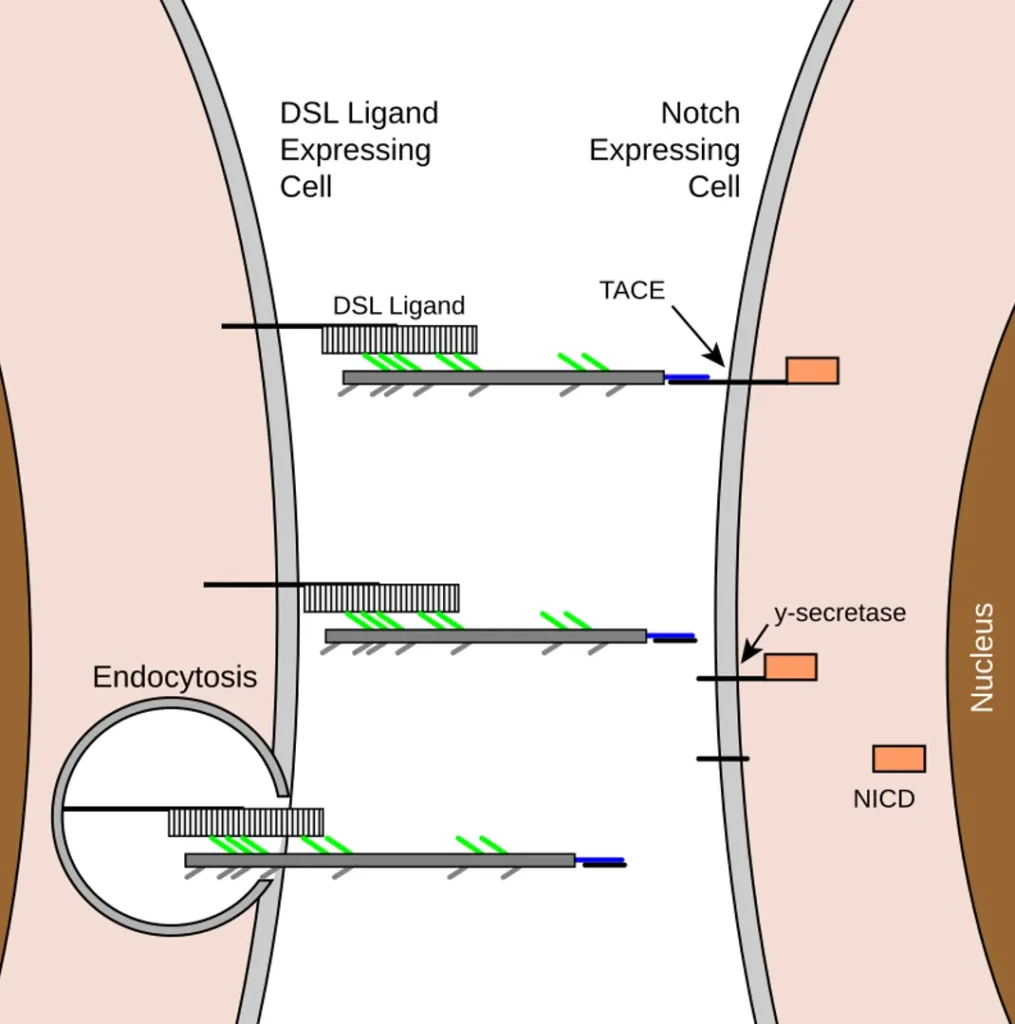
Consider embryonic nervous system development in fruit flies or vertebrates. If a cell expresses high levels of Delta, it signals neighbors via Notch to avoid becoming neurons, promoting diversity in cell types. This lateral inhibition prevents all cells in a group from adopting the same role, creating patterns like spaced-out neurons amid supportive glial cells.
Such interactions are bidirectional at times, with signals flowing both ways, adding layers of complexity to tissue organization.
Type 2: Communicating Junctions and Direct Cytoplasmic Links
Gap junctions exemplify this type in animal cells. Composed of connexin proteins, these channels form pores connecting adjacent cells’ cytoplasms, allowing small molecules like ions, ATP, or cyclic AMP to pass through. It’s like cells sharing a secret passage for quick exchanges.
In heart muscle cells, or cardiomyocytes, gap junctions synchronize contractions by propagating electrical signals, ensuring the heart beats as a unified pump. Disruptions here can lead to arrhythmias. Similarly, in early embryos, gap junctions create compartments where cells communicate to coordinate development. For example, in frog embryos, blocking these junctions with antibodies causes severe defects, like malformed tadpoles.
In plants, plasmodesmata serve a similar role, bridging cell walls to allow symplastic transport of signals. These channels can adjust their size to regulate what passes, playing a part in defense responses, such as spreading signals during pathogen attacks.
Type 3: Extracellular Matrix Interactions
The extracellular matrix isn’t just structural; it’s a signaling hub. Glycoproteins like fibronectin or laminin bind to integrins on cell surfaces, influencing migration, survival, and differentiation.
During development, fibronectin guides migrating cells, such as heart precursors in chick embryos. If antibodies block this interaction, cells fail to reach their destinations, resulting in abnormalities like two separate hearts. In mammary glands, laminin-integrin binding promotes milk protein production, halting cell division and fostering specialized tissues.
This type also prevents apoptosis, or programmed cell death. Cells detached from the matrix, like in cancer metastasis, often die unless they adapt, highlighting the survival signals provided by these interactions.
How Juxtacrine Signaling Works
Understanding the mechanics reveals why juxtacrine signaling is so effective. It starts with ligand-receptor binding at the cell interface. For membrane-bound types, this triggers intracellular cascades, like enzyme activation or gene transcription.
In the Notch pathway, binding leads to proteolytic cleavage of Notch, freeing the intracellular domain to act as a transcription factor. This can activate genes for differentiation or inhibit them, depending on context.
For gap junctions, molecules diffuse passively, creating waves of activity, such as calcium ions coordinating muscle contractions or neuronal firing.
Matrix interactions often involve integrins linking to the cytoskeleton, transmitting mechanical forces that influence cell shape and function. This mechanotransduction converts physical cues into biochemical signals, blending structure and signaling.
These mechanisms ensure rapid, specific responses, often irreversible, like committing a cell to a particular fate.
Real-World Examples of Juxtacrine Signaling in Animal Development
Development is where juxtacrine signaling shines, orchestrating the transformation from a single cell to a complex organism.
In neural development, the Notch-Delta system ensures proper spacing of neurons. In Drosophila, proneural cells express Delta, inhibiting neighbors from becoming neurons, resulting in a balanced network. In vertebrates, it decides between neuronal and glial fates in the eye and brain.
During organogenesis, like heart formation, gap junctions and matrix signals guide cells. Connexin-43 mutations in mice cause heart defects, showing its critical role.
In the intestine, Notch signaling directs stem cells toward absorptive or secretory lineages, maintaining gut homeostasis. Without it, imbalances could lead to disorders like cancer.
These examples illustrate how juxtacrine signaling builds diversity and order in tissues.
Juxtacrine Signaling in Plant Development and Defense
Though often associated with animals, juxtacrine signaling thrives in plants via plasmodesmata. These channels allow viruses, RNAs, and proteins to move between cells, influencing growth and responses.
In defense, signals like azelaic acid travel through plasmodesmata to trigger systemic acquired resistance, preparing distant tissues against pathogens.
During development, plasmodesmata regulate symplastic domains, controlling where signals spread to shape organs like leaves or roots. Their dynamic nature, widening or narrowing, adds regulatory finesse.
This highlights evolutionary conservation of contact-dependent communication across kingdoms.
The Crucial Role of Juxtacrine Signaling in the Immune Response
In immunity, juxtacrine signaling enables precise activation and regulation, preventing overreactions.
A prime example is T-cell activation. Antigen-presenting cells display peptides on MHC molecules, binding T-cell receptors in a juxtacrine manner. This contact initiates immune attacks on infected cells.
Checkpoint pathways like PD-1/PD-L1 involve juxtacrine interactions where tumor cells upregulate PD-L1 to suppress T-cells, evading immunity. Therapies targeting these have revolutionized cancer treatment.
Neutrophil extravasation uses juxtacrine signals between endothelial cells and neutrophils, allowing white blood cells to roll, adhere, and exit vessels toward infection sites.
CTLA-4 on T-cells binds ligands on antigen-presenting cells, dampening responses to avoid autoimmunity.
These interactions ensure the immune system is targeted and controlled.
Juxtacrine Signaling in Unicellular Organisms Like Bacteria
Even in single-celled life, juxtacrine signaling occurs through membrane contacts. In bacteria, it involves direct interactions for behaviors like biofilm formation or conjugation.
During conjugation, bacteria connect via pili to exchange genetic material, a form of contact-dependent signaling that promotes antibiotic resistance spread.
In biofilms, cells touch to coordinate quorum sensing-like responses, though traditionally diffusive, some involve direct membrane fusion or nanotubes.
This suggests juxtacrine signaling is an ancient mechanism, predating multicellularity.
Comparing Juxtacrine Signaling to Other Cell Communication Methods
To appreciate juxtacrine signaling, let’s contrast it with others. Each type suits different needs, from local to systemic control.
Here’s a detailed comparison table:
| Signaling Type | Distance | Mechanism | Key Examples | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Autocrine | Self | Cell signals itself via released factors | Cancer cells stimulating own growth | Self-regulation | Can lead to uncontrolled proliferation |
| Paracrine | Short | Diffusible signals to nearby cells | Neurotransmitters in synapses | Quick local effects | Signal dilution over distance |
| Endocrine | Long | Hormones via bloodstream | Insulin regulating blood sugar | Body-wide coordination | Slower response time |
| Juxtacrine | Contact | Direct membrane or matrix interaction | Notch-Delta in development | High specificity, no diffusion loss | Limited to adjacent cells |
| Synaptic | Targeted | Electrical/chemical at synapses | Neuron communication | Ultra-fast transmission | Requires specialized structures |
This table shows how juxtacrine signaling fills a niche for precise, contact-based interactions.
Key Molecules Involved in Juxtacrine Signaling
Many molecules drive juxtacrine signaling. Below is an extensive table listing ligands, receptors, and their roles:
| Molecule Category | Specific Examples | Role in Signaling | Associated Processes | Organisms Involved |
|---|---|---|---|---|
| Ligands | Delta, Jagged, Serrate | Bind to Notch receptors | Cell fate determination, lateral inhibition | Animals, including flies and vertebrates |
| Fibronectin, Laminin | Interact with integrins | Cell migration, adhesion, survival | Mammals, birds | |
| MHC-peptide complexes | Bind T-cell receptors | Immune activation | Humans, mammals | |
| PD-L1 | Binds PD-1 on T-cells | Immune checkpoint, suppression | Cancer cells, immune cells | |
| Receptors | Notch | Cleaved upon binding, activates transcription | Development, immunity | Eukaryotes |
| Integrins | Link matrix to cytoskeleton | Mechanotransduction, differentiation | Animals, plants (similar) | |
| Connexins | Form gap junctions | Ion and molecule passage | Animals | |
| T-cell receptors | Recognize antigens | Pathogen response | Vertebrates | |
| Junction Structures | Gap junctions | Direct cytoplasmic links | Synchronization in heart, embryos | Animals |
| Plasmodesmata | Symplastic channels | Defense, development | Plants |
This table captures the diversity of players in juxtacrine signaling.
The Importance of Juxtacrine Signaling in Health and Disease
Beyond normal function, disruptions in juxtacrine signaling underlie many diseases. Mutations in Notch pathways link to cancers like T-cell leukemia, where unchecked signaling promotes tumor growth.
In heart diseases, connexin defects cause arrhythmias, impairing synchronized beating.
Immune disorders, like autoimmune diseases, may stem from faulty checkpoints like CTLA-4, leading to self-attacks.
In development, errors can cause congenital anomalies, such as improper organ formation.
Understanding these has led to therapies, like integrin-targeted drugs for inflammation or Notch inhibitors for cancer.
Historical Background and Discovery
The term juxtacrine was coined in 1990 by Anklesaria and colleagues to describe TGF-alpha and EGFR interactions. This built on earlier studies of cell contact in development and immunity.
Since then, research has exploded, with the Notch pathway discovered in the 1980s becoming a cornerstone.
Advances in imaging and genetics have revealed its nuances, from bacteria to humans.
In summary, juxtacrine signaling is a foundational process that ensures cells communicate effectively through touch. From shaping embryos to fighting infections, its roles are vast and vital. As research progresses, we’ll uncover more about its potential in medicine, perhaps leading to new treatments for developmental disorders or immune-related conditions. This direct dialogue between cells reminds us of the intricate teamwork underlying life itself.
Frequently Asked Questions
FAQ 1: What is juxtacrine signaling and how does it work?
Juxtacrine signaling is a fascinating way cells chat with each other, but only when they’re touching directly, like close friends whispering secrets. Unlike other signaling methods where messages float through fluids, this one keeps everything up close and personal.
Basically, a cell uses a molecule stuck on its surface, called a ligand, to bind with a receptor on the neighboring cell’s surface. This binding kicks off a chain reaction inside the receiving cell, changing how it behaves, grows, or even decides what type of cell it becomes. It’s super precise because there’s no chance for the signal to wander off and affect the wrong cells, which makes it perfect for detailed work in the body, like building tissues or fighting off invaders.
The process starts at the cell membrane where the ligand and receptor meet. Once they connect, it can trigger things like enzyme activity or gene changes in the nucleus. For example, in some cases, the receptor gets chopped up, and a piece travels inside to switch genes on or off. This happens quickly and stays local, avoiding any mix-ups. Researchers have seen this in action with proteins like cytokines that help immune cells team up during infections. It’s also key in development, where one cell tells another to become a specific type, ensuring everything forms just right.
But it’s not all about membranes touching. Sometimes, the extracellular matrix, that supportive goo around cells, gets involved by providing ligands that hook onto cell receptors. Or, tiny channels called gap junctions let small molecules zip directly from one cell’s insides to another’s. These variations make juxtacrine signaling versatile, handling everything from heartbeats syncing up to bacteria swapping genes. Overall, it’s a cornerstone of how life organizes itself, from simple organisms to complex humans, keeping communications tight and targeted.
FAQ 2: How does juxtacrine signaling differ from paracrine signaling?
When comparing juxtacrine signaling and paracrine signaling, the big standout is distance and delivery. Juxtacrine requires cells to be in direct contact, like a firm handshake, where signals pass through surface molecules without leaving the cells. Paracrine, on the other hand, lets cells send messages over short distances by releasing chemicals into the surrounding fluid, which then diffuse to nearby cells. This makes paracrine more like tossing a note across a room, quick but potentially less precise if the note drifts.
Both play huge roles in the body, but their differences affect how they’re used. For instance, juxtacrine is ideal for situations needing exact control, such as in embryo patterning where one cell directly influences its neighbor’s fate. Paracrine shines in rapid local responses, like inflammation where cells release factors to recruit help from a bit farther away.
Here are some key contrasts to break it down further:
- Range of action: Juxtacrine is strictly contact-dependent, limiting it to adjacent cells, while paracrine can reach cells a short distance away through diffusion.
- Signal molecule behavior: In juxtacrine, ligands stay bound to the sending cell or matrix; in paracrine, they’re secreted and float freely.
- Speed and specificity: Juxtacrine offers high specificity with minimal loss, but it’s slower to set up without contact. Paracrine is faster for group alerts but risks dilution or off-target effects.
- Examples in biology: Juxtacrine includes Notch interactions in development; paracrine involves neurotransmitters at synapses or growth factors in wound healing.
- Evolutionary perks: Juxtacrine might be more ancient, seen even in bacteria, whereas paracrine allows for more flexible community responses in multicellular life.
These differences mean the body picks the right method for the job, blending them for seamless coordination.
FAQ 3: What are the three main types of juxtacrine signaling?
| Type of Juxtacrine Signaling | Description | Key Mechanisms Involved | Biological Examples | Importance in Organisms |
|---|---|---|---|---|
| Membrane-bound ligand and receptor interaction | This involves a ligand like a protein or lipid on one cell’s surface binding directly to a receptor on an adjacent cell, triggering internal changes without the ligand detaching. | Binding causes receptor activation, often leading to cleavage and nuclear signaling. | Notch-Delta pathway in neural development, where Delta on one cell inhibits neuron formation in neighbors. | Crucial for cell fate decisions in embryos, ensuring diverse cell types emerge properly. |
| Communicating junctions for intracellular links | Here, channels like gap junctions connect the cytoplasms of two cells, allowing small molecules such as ions or ATP to pass directly. | Passive diffusion through pores formed by proteins like connexins. | Synchronization of heart muscle contractions in cardiomyocytes to maintain rhythm. | Essential for coordinated activities in tissues like the heart or early embryos, preventing disorders like arrhythmias. |
| Extracellular matrix glycoprotein and membrane protein interaction | Ligands in the supportive matrix around cells bind to receptors on cell surfaces, influencing behavior without cell-to-cell contact. | Integrins link matrix components like fibronectin to the cell’s cytoskeleton, transmitting mechanical signals. | Guiding cell migration during wound healing or organ formation, such as in chick heart development. | Vital for tissue structure and repair, with disruptions linked to issues like cancer metastasis or developmental defects. |
This table highlights how each type adapts juxtacrine signaling for specific needs, from direct chats to matrix-guided cues, drawing from observations in various organisms.
FAQ 4: How does the Notch pathway exemplify juxtacrine signaling?
The Notch pathway is a prime example of juxtacrine signaling because it relies entirely on direct cell contact to function. It starts when a ligand like Delta or Jagged on one cell’s membrane binds to the Notch receptor protruding from a neighboring cell. This binding isn’t just a simple lock-and-key; it pulls on the receptor, causing it to be cleaved by enzymes. A piece of the receptor then breaks free and heads to the nucleus, where it teams up with other proteins to turn genes on or off. This process, called lateral inhibition, helps create patterns in tissues by making sure not all cells in a group do the same thing.
In development, this pathway shines in creating diversity. Take the nervous system: if one cell ramps up Delta, it signals its neighbors via Notch to hold back from becoming neurons, pushing them toward supportive roles like glial cells. This spacing out prevents overcrowding and builds a balanced network. It’s seen in fruit flies’ eyes or vertebrate brains, where without it, things could get chaotic, leading to too many or too few of certain cells.
Beyond development, the Notch pathway pops up in adult tissues too, like maintaining stem cells in the gut or regulating blood vessel formation. But when it goes wrong, it can fuel diseases such as certain leukemias, where overactive signaling promotes uncontrolled growth. Therapies targeting this pathway aim to dial it back, showing how understanding juxtacrine mechanics could lead to medical breakthroughs. Overall, the Notch pathway shows juxtacrine signaling’s power in precise, contact-based control.
FAQ 5: What role does juxtacrine signaling play in embryonic development?
Juxtacrine signaling is like the director in the early stages of life, guiding cells on what to become and where to go. It ensures that as an embryo grows from a blob of cells into something structured, everything happens in the right order. By requiring direct contact, it prevents signals from scattering, allowing for pinpoint accuracy in building organs and tissues.
Key ways it contributes include:
- Cell fate determination: Signals like those in the Notch pathway tell cells to differentiate into specific types, such as skin or muscle, creating diversity from identical starting points.
- Patterning and organization: It helps form patterns, like spacing out hair follicles or neurons, through processes like lateral inhibition where one cell influences its neighbors’ choices.
- Organogenesis support: In forming hearts or kidneys, juxtacrine cues guide cell migration and adhesion, ensuring parts connect properly.
- Tissue boundary formation: It sets up borders between different tissue types, preventing mixing that could lead to defects.
- Coordination via junctions: Gap junctions allow rapid sharing of ions, syncing cell activities during critical phases like gastrulation.
Without this signaling, embryos might develop abnormalities, highlighting its foundational role in life’s blueprint.
FAQ 6: How is juxtacrine signaling involved in the immune system?
| Aspect of Immune Involvement | Description | Key Molecules or Pathways | Examples in Action | Potential Implications for Health |
|---|---|---|---|---|
| T-cell activation | Direct contact between antigen-presenting cells and T-cells triggers immune responses through surface bindings. | MHC-peptide complexes binding to T-cell receptors. | Recognition and attack on infected cells during viral infections. | Disruptions can lead to weakened defenses against pathogens. |
| Immune checkpoint regulation | Contact-dependent signals suppress overactive responses to prevent autoimmunity. | PD-1/PD-L1 interactions where tumor cells exploit this to evade attack. | Cancer cells upregulating PD-L1 to inhibit T-cell killing. | Basis for checkpoint inhibitor therapies in oncology. |
| Neutrophil recruitment | Endothelial cells signal neutrophils to adhere and exit blood vessels toward infection sites. | Selectins and integrins facilitating rolling and adhesion. | Inflammation response in bacterial infections. | Faulty signaling contributes to chronic inflammation or poor wound healing. |
| Macrophage-tumor interactions | Macrophages contact cancer cells, sometimes promoting resistance to treatments. | Ephrin receptors influencing PD-L1 expression. | Tumor-associated macrophages shielding cancer from radiation. | Targets for enhancing cancer therapies by blocking these contacts. |
| Overall immune coordination | Juxtacrine ensures targeted activation, avoiding widespread inflammation. | Cytokines and chemokines in contact form. | B-cell and T-cell collaborations in lymph nodes. | Understanding this could improve vaccines or treat autoimmune diseases. |
This table shows juxtacrine’s critical, contact-specific roles in immunity, blending precision with power.
FAQ 7: Is juxtacrine signaling present in plants, and if so, how?
Yes, juxtacrine signaling does occur in plants, though it’s adapted to their unique structure with cell walls. Instead of direct membrane touches like in animals, plants use channels called plasmodesmata that pierce through walls, connecting cytoplasms of adjacent cells. These act like tunnels for small molecules, proteins, or even RNAs to pass, enabling contact-dependent communication without the cells merging.
In plant development, this signaling helps shape leaves, roots, and flowers by controlling where signals spread. For example, during organ formation, plasmodesmata regulate symplastic domains, deciding which groups of cells share information. This creates patterns, like vein layouts in leaves, ensuring nutrients and signals flow correctly.
Defense is another big area. When a pathogen attacks, signals like reactive oxygen species or hormones zip through plasmodesmata to alert neighbors, triggering responses like thickening walls or producing toxins. These channels can even adjust size to block viruses while allowing helpful molecules through.
Overall, while animal juxtacrine often involves ligands and receptors, plant versions lean on these physical links, showing evolution’s clever twists on the same idea for walled cells.
FAQ 8: What diseases are linked to disruptions in juxtacrine signaling?
Disruptions in juxtacrine signaling can throw off the body’s balance, leading to various diseases by messing with cell communication. In cancer, for instance, faulty Notch pathways cause cells to grow unchecked, as seen in T-cell leukemias where overactive signals ignore normal stop signs.
Heart issues arise too, like arrhythmias from mutated connexins in gap junctions, which fail to sync beats properly, risking sudden cardiac events.
In the immune realm, problems with checkpoints like PD-1/PD-L1 let tumors hide, or conversely, trigger autoimmunity if signals don’t dampen responses.
Developmental disorders stem from early glitches, such as in osteogenesis imperfecta linked to Wnt1 mutations affecting bone formation.
Even in the brain, faulty signaling contributes to conditions like intellectual disabilities by impairing blood-brain barrier integrity.
These links show how vital juxtacrine is, with research eyeing fixes like targeted drugs to restore order.
FAQ 9: Who discovered juxtacrine signaling and when?
The concept of juxtacrine signaling was first coined in 1990 by researchers led by Perviz Anklesaria, who were studying how transforming growth factor alpha (TGF-alpha) interacts with epidermal growth factor receptors (EGFR). They noticed that some signals didn’t diffuse but stayed membrane-bound, prompting them to describe this contact-dependent method. This built on earlier observations of cell interactions in development and immunity, but the term gave it a clear identity.
Before that, groundwork came from studies in the 1980s on pathways like Notch, discovered in fruit flies, showing how ligands and receptors on adjacent cells communicate. By the 1990s, it was clear this wasn’t just paracrine or endocrine but a distinct category.
The discovery opened doors to understanding diseases and therapies, as scientists realized many processes rely on this intimate signaling. It’s evolved since, with modern tools revealing its noise and nuances, but that 1990 paper marked the start.
FAQ 10: What are potential future applications of juxtacrine signaling in medicine?
| Application Area | Description | Potential Benefits | Challenges to Overcome | Emerging Research Examples |
|---|---|---|---|---|
| Cancer therapy enhancement | Targeting juxtacrine pathways like Notch or PD-L1 to block tumor evasion or growth. | Improved immune attacks on cancers, reducing resistance to treatments like radiation. | Avoiding side effects on normal cells; precise delivery. | Inhibitors for ephrin signaling to lower PD-L1 in tumors. |
| Regenerative medicine | Using synthetic cells or engineered signaling to repair tissues via contact-dependent cues. | Faster wound healing or organ regeneration by mimicking natural development. | Ensuring biocompatibility; controlling signal strength. | Light-activated synthetic cells for guided tissue growth. |
| Immune modulation | Manipulating checkpoints for autoimmune diseases or vaccines. | Better control of overactive immunity or boosted responses to infections. | Balancing suppression and activation to prevent new issues. | Programmable receptors in therapeutic cells for targeted responses. |
| Cardiovascular treatments | Fixing gap junction defects to restore heart rhythm. | Preventing arrhythmias through gene therapies or drugs. | Long-term safety in dynamic heart tissue. | Connexin modulators for congenital heart defects. |
| Neurological disorders | Enhancing blood-brain barrier integrity via cadherin signaling. | Treating conditions like Alzheimer’s by reducing leakage and neurotoxicity. | Crossing the barrier for drug delivery. | N-cadherin boosts to improve cognitive function in models. |
This table outlines promising paths, blending current insights with innovative tech for healthier futures.
FAQ 11: How does juxtacrine signaling differ from autocrine signaling?
Juxtacrine signaling and autocrine signaling are both ways cells communicate, but they operate on different scales and with unique mechanics that suit specific biological needs. In juxtacrine signaling, everything happens through direct contact, where a ligand on one cell’s surface binds to a receptor on a neighboring cell, ensuring the message stays pinpointed and doesn’t spread out. This makes it ideal for situations requiring precision, like guiding cell fates in developing tissues or coordinating immune responses. Autocrine signaling, by contrast, involves a cell sending a signal to itself, releasing molecules that loop back to its own receptors, often to reinforce its behavior or growth.
The key difference lies in the target and delivery. Juxtacrine demands physical closeness, preventing signals from affecting distant cells and minimizing errors in crowded environments. Autocrine allows a cell to self-regulate without needing others, which can be handy for quick adjustments but risks creating loops that lead to issues like uncontrolled proliferation in diseases. For example, in wound healing, juxtacrine might help adjacent cells team up to close the gap, while autocrine could spur a single cell to divide rapidly on its own.
These distinctions highlight how the body mixes signaling types for efficiency. While juxtacrine promotes teamwork among neighbors, autocrine focuses on independence, and both can overlap with other methods like paracrine for broader effects. Understanding these helps explain why disruptions in one might not affect the other, offering clues for targeted therapies in conditions where cell communication goes awry.
FAQ 12: What is the role of juxtacrine signaling in cancer development and progression?
Juxtacrine signaling plays a sneaky yet powerful role in cancer, often helping tumors grow, evade the immune system, and spread by enabling direct cell chats that normal cells use for good. In healthy tissues, this signaling keeps things organized, but in cancer, it gets hijacked. Tumor cells might use it to signal nearby fibroblasts or immune cells, creating a supportive environment that shields them from attacks or promotes new blood vessels.
Key ways it contributes include:
- Boosting tumor survival: Direct contacts upregulate proteins like PD-L1 on cancer cells, telling immune cells to back off and allowing tumors to dodge detection.
- Encouraging metastasis: By interacting with the extracellular matrix or adjacent cells, juxtacrine cues guide cancer cells to break away and invade new areas, much like directing traffic in a busy city.
- Influencing the microenvironment: Tumor-associated macrophages use juxtacrine to protect cancer stem cells, forming a niche that resists treatments like chemotherapy.
- Promoting resistance: In breast or ovarian cancers, these signals make cells tougher against radiation or drugs by inhibiting cell death pathways.
- Driving uncontrolled growth: Pathways like Notch get overactive, pushing cells to divide endlessly without the usual checks.
This makes juxtacrine a double-edged sword, essential for development but dangerous when altered. Researchers are eyeing it for new treatments, like blocking specific interactions to starve tumors of their supportive network.
FAQ 13: How does juxtacrine signaling occur in bacteria and other unicellular organisms?
| Aspect of Juxtacrine in Unicellular Life | Description | Mechanisms Involved | Examples from Organisms | Biological Significance |
|---|---|---|---|---|
| Direct membrane contact | In single-celled organisms, signaling happens when cells touch, exchanging info without releasing molecules into the environment. | Membrane proteins or structures like pili bind, allowing gene or signal transfer. | Bacterial conjugation where DNA passes through pili during mating. | Enables genetic diversity and adaptation, like spreading antibiotic resistance. |
| Biofilm coordination | Cells in communities use contact to synchronize behaviors, forming protective layers. | Surface receptors detect neighbors, triggering collective responses. | Pseudomonas aeruginosa in biofilms sharing nutrients or defense signals. | Helps survival in harsh conditions, contributing to chronic infections. |
| Quorum sensing integration | While often diffusive, juxtacrine adds a contact layer for precise local control. | Nanotubes or direct fusion connect cytoplasms for molecule exchange. | Myxobacteria gliding together to form fruiting bodies during starvation. | Promotes group decisions, like sporulation or virulence activation. |
| Evolutionary precursors | This signaling likely predates multicellularity, serving as a building block for complex life. | Ancient proteins similar to eukaryotic receptors facilitate interactions. | Yeast mating where cells touch to fuse and exchange nuclei. | Shows conservation across domains, aiding study of communication origins. |
| Stress response sharing | Contact allows rapid alert to environmental threats among clustered cells. | Ion or small molecule passage through channels. | E. coli colonies signaling via membrane contacts to resist antibiotics. | Enhances population resilience, impacting medical and ecological fields. |
This table illustrates how juxtacrine adapts to simple life forms, emphasizing its ancient roots and practical roles.
FAQ 14: What are the details of gap junctions in juxtacrine signaling?
Gap junctions are like secret tunnels between cells, forming a key part of juxtacrine signaling by allowing direct sharing of small molecules without any outside travel. Made up of proteins called connexins, these junctions create pores that connect the insides of adjacent cells, letting ions, nucleotides, and other tiny signals zip through. This setup is perfect for quick synchronization, such as in heart tissue where it ensures beats happen in unison, preventing chaos like irregular rhythms.
In the nervous system, gap junctions help neurons communicate electrically, speeding up signals in ways chemical synapses can’t match. They’re not static either; cells can open or close these channels based on needs, like during development when embryos need coordinated growth waves. Disruptions, such as mutations in connexin genes, can lead to issues ranging from deafness to skin disorders, showing how vital this direct link is.
Beyond animals, plants have similar structures called plasmodesmata, which bridge cell walls for symplastic communication, underlining the evolutionary importance of these junctions. Overall, gap junctions make juxtacrine signaling efficient and versatile, blending electrical and chemical cues for seamless cellular teamwork.
FAQ 15: What is the role of the extracellular matrix in juxtacrine signaling?
The extracellular matrix acts as a supportive scaffold that also dives into signaling, making it a crucial player in one type of juxtacrine communication. It’s not just passive glue; it provides ligands like glycoproteins that bind to cell surface receptors, influencing everything from movement to survival without needing cell-to-cell touch.
Important contributions include:
- Guiding cell behavior: Components like fibronectin hook onto integrins, pulling cells in directions needed for tissue formation or repair.
- Transmitting mechanical cues: The matrix converts physical forces into biochemical signals, helping cells sense stiffness and adapt, which is key in wound healing.
- Regulating growth factors: It stores and releases signals, fine-tuning juxtacrine effects in stem cell niches.
- Preventing cell death: Anchorage-dependent signals stop apoptosis, ensuring cells stay put and functional.
- Influencing disease: In cancer, altered matrix interactions help tumors invade, highlighting potential therapy spots.
This integration shows the matrix as a dynamic partner in juxtacrine, blending structure with communication for healthy tissues.
FAQ 16: How does juxtacrine signaling compare to endocrine signaling?
| Comparison Aspect | Juxtacrine Signaling | Endocrine Signaling | Key Differences | Shared Features |
|---|---|---|---|---|
| Distance of action | Requires direct cell contact or matrix proximity. | Travels long distances via bloodstream. | Juxtacrine is local; endocrine is systemic. | Both use ligands to bind receptors. |
| Speed of response | Rapid due to immediate binding. | Slower as hormones circulate body-wide. | Juxtacrine suits quick local fixes; endocrine for broad regulation. | Involve signal amplification inside cells. |
| Signal molecule type | Membrane-bound ligands or junction molecules. | Hormones like insulin released into blood. | Juxtacrine avoids diffusion; endocrine relies on it. | Can trigger gene expression changes. |
| Biological examples | Notch pathway in development or gap junctions in heart. | Thyroid hormones controlling metabolism. | Juxtacrine for tissue patterning; endocrine for homeostasis. | Essential in multicellular coordination. |
| Evolutionary role | Ancient, seen in bacteria; precise control. | More advanced for complex organisms. | Juxtacrine for fine-tuned interactions; endocrine for integration. | Both adapt to environmental cues. |
This table breaks down the contrasts, showing how each fits different biological niches.
FAQ 17: What is the evolutionary history of juxtacrine signaling?
Juxtacrine signaling likely emerged early in life’s timeline, serving as a basic way for cells to interact before complex multicellularity took hold. In ancient unicellular organisms, like bacteria, it allowed simple contacts for gene exchange or community coordination, laying groundwork for more sophisticated systems. As life evolved, this direct method adapted, becoming crucial in eukaryotes for precise developmental cues.
Over time, it conserved core features across kingdoms, from bacterial pili to animal Notch pathways, suggesting strong selective pressure for reliability. In plants, plasmodesmata evolved to bridge walls, while animals refined gap junctions for electrical sync. This progression enabled the rise of tissues and organs, where juxtacrine ensures order amid chaos.
Today, its role in diseases underscores evolutionary trade-offs, where mechanisms for growth can turn harmful. Studying this history reveals how simple touches built life’s complexity.
FAQ 18: What techniques are used to study juxtacrine signaling?
Scientists employ a mix of clever methods to peek into juxtacrine signaling, combining biology with tech for clear insights. These approaches help visualize and manipulate the direct cell interactions that are hard to catch otherwise.
Common techniques include:
- Fluorescent labeling and imaging: Tagging ligands or receptors with glowing proteins lets researchers watch bindings in real-time under microscopes.
- Co-culture systems: Growing different cells together mimics natural contacts, allowing study of signals like in vascular or immune setups.
- Synthetic gene circuits: Engineering cells with custom pathways reveals how contact area or growth affects signaling.
- Optogenetics: Using light to activate or block interactions provides precise control over when and where signals happen.
- Proximal culture assays: Separating cells with filters isolates juxtacrine from other signals, pinpointing direct effects.
These tools advance our grasp, paving ways for medical applications.
FAQ 19: How is juxtacrine signaling targeted in therapies?
| Therapeutic Target | Description | Potential Benefits | Challenges | Examples of Approaches |
|---|---|---|---|---|
| Notch pathway inhibition | Blocking direct ligand-receptor binds in overactive cancers. | Halts tumor growth and boosts immune response. | Off-target effects on healthy development. | Gamma-secretase inhibitors in trials. |
| Checkpoint modulation | Disrupting PD-L1 contacts to unleash T-cells. | Enhances antitumor immunity in resistant cases. | Balancing to avoid autoimmunity. | Antibodies targeting ephrin receptors. |
| Macrophage-tumor interactions | Interfering with signals that protect cancer from treatments. | Improves radiation or chemo efficacy. | Specificity in dynamic environments. | Drugs blocking juxtacrine-induced resistance. |
| Synthetic signaling mimics | Engineering cells to deliver controlled juxtacrine cues. | Aids tissue repair or regeneration. | Ensuring safety and integration. | Light-activated systems for wound healing. |
| Growth factor delivery | Using artificial setups for localized signaling. | Controls cell function in grafts or implants. | Long-term stability. | Targeted systems for bone or vascular therapy. |
This table spotlights emerging strategies, blending disruption with enhancement for better outcomes.
FAQ 20: What role does juxtacrine signaling play in neural function and the nervous system?
Juxtacrine signaling is vital in the nervous system, acting as a precise coordinator from early development to adult function. It guides neuron formation by deciding fates through contacts like the Notch-Delta interaction, ensuring a balanced mix of cell types without overcrowding. This lateral inhibition creates patterns, spacing out neurons amid supportive cells for efficient wiring.
In mature brains, it supports synaptic plasticity and communication via gap junctions, allowing electrical coupling that speeds signals in networks like those for reflexes or rhythms. Disruptions can impair guidance or repair, linking to disorders like neurodegeneration.
Overall, its direct nature makes the nervous system robust yet vulnerable, with ongoing research exploring fixes for conditions like epilepsy.
Acknowledgement
The Examsmeta.com website expresses its gratitude to the scientific community and various online resources that have significantly enriched the content of the article “Juxtacrine Signaling: Mechanisms, Functions, and Biological Roles.” The insights drawn from these reputable platforms have been instrumental in providing accurate, up-to-date, and comprehensive information on the intricate mechanisms of juxtacrine signaling. Their dedication to disseminating high-quality biological research has greatly contributed to the depth and clarity of this article, ensuring it serves as a valuable resource for readers seeking to understand this critical cellular process.
Key resources include:
- Nature (www.nature.com) for its extensive publications on cell signaling pathways, particularly the Notch pathway and its role in development and disease.
- ScienceDirect (www.sciencedirect.com) for providing detailed studies on gap junctions and extracellular matrix interactions in various organisms.
- PubMed (pubmed.ncbi.nlm.nih.gov) for offering access to peer-reviewed articles on immune responses and bacterial signaling mechanisms.
- Cell (www.cell.com) for its in-depth reviews on cancer biology and the role of juxtacrine signaling in tumor microenvironments.
- Journal of Biological Chemistry (www.jbc.org) for foundational research on ligand-receptor interactions and their evolutionary significance.

