Mitochondria are often called the powerhouses of the cell because they play a central role in generating energy through processes like ATP synthesis. However, this energy production comes with a downside: the creation of harmful byproducts known as reactive oxygen species (ROS). These molecules, while sometimes beneficial in small amounts for signaling and immune responses, can cause significant damage when they accumulate. That’s where manganese superoxide dismutase (MnSOD) steps in as a crucial defender. This enzyme, located primarily in the mitochondrial matrix, helps neutralize superoxide radicals, preventing oxidative harm to cellular components.
In this article, we’ll explore the intricate world of MnSOD, its role in combating ROS, how imbalances lead to diseases, and emerging ways to harness this knowledge for better health outcomes.
Table of Contents
The mitochondrion isn’t just an energy factory; it’s a hub for numerous metabolic pathways. For instance, it hosts key steps in the breakdown of fatty acids through β-oxidation, the conversion of waste products in the urea cycle, and the turning of the citric acid cycle wheels to produce energy precursors. But amid all this activity, ROS emerge as inevitable side effects, mainly from the electron transport chain where oxygen is reduced. When ROS levels spike abnormally, they can oxidize proteins, lipids, and DNA within the mitochondria, disrupting these vital functions. This oxidative stress has been linked to a wide array of health issues, from aging to chronic diseases. Understanding MnSOD‘s protective mechanisms offers insights into maintaining cellular balance and could pave the way for novel therapies.
ROS aren’t all bad—they serve as signaling molecules that help regulate cell growth, differentiation, and even programmed cell death, or apoptosis. For example, in the immune system, ROS aid in fighting off pathogens by activating inflammatory responses. However, the line between helpful and harmful is thin. When production outpaces elimination, ROS can damage mitochondrial DNA, which lacks the robust repair systems found in nuclear DNA, leading to mutations and further dysfunction. This delicate balance underscores why enzymes like MnSOD are indispensable. By converting superoxide into less reactive forms, MnSOD helps preserve mitochondrial integrity and overall cell health.
Mitochondrial Production of ROS
Mitochondria stand out as the primary generators of ROS in most cells, largely because they consume the bulk of cellular oxygen during respiration. The electron transport chain (ETC), embedded in the inner mitochondrial membrane, is where electrons from nutrients like glucose or fats are passed along to create a proton gradient for ATP production. But not all electrons make it smoothly to their final destination—some leak out and react with oxygen to form superoxide radicals (O₂⁻•).
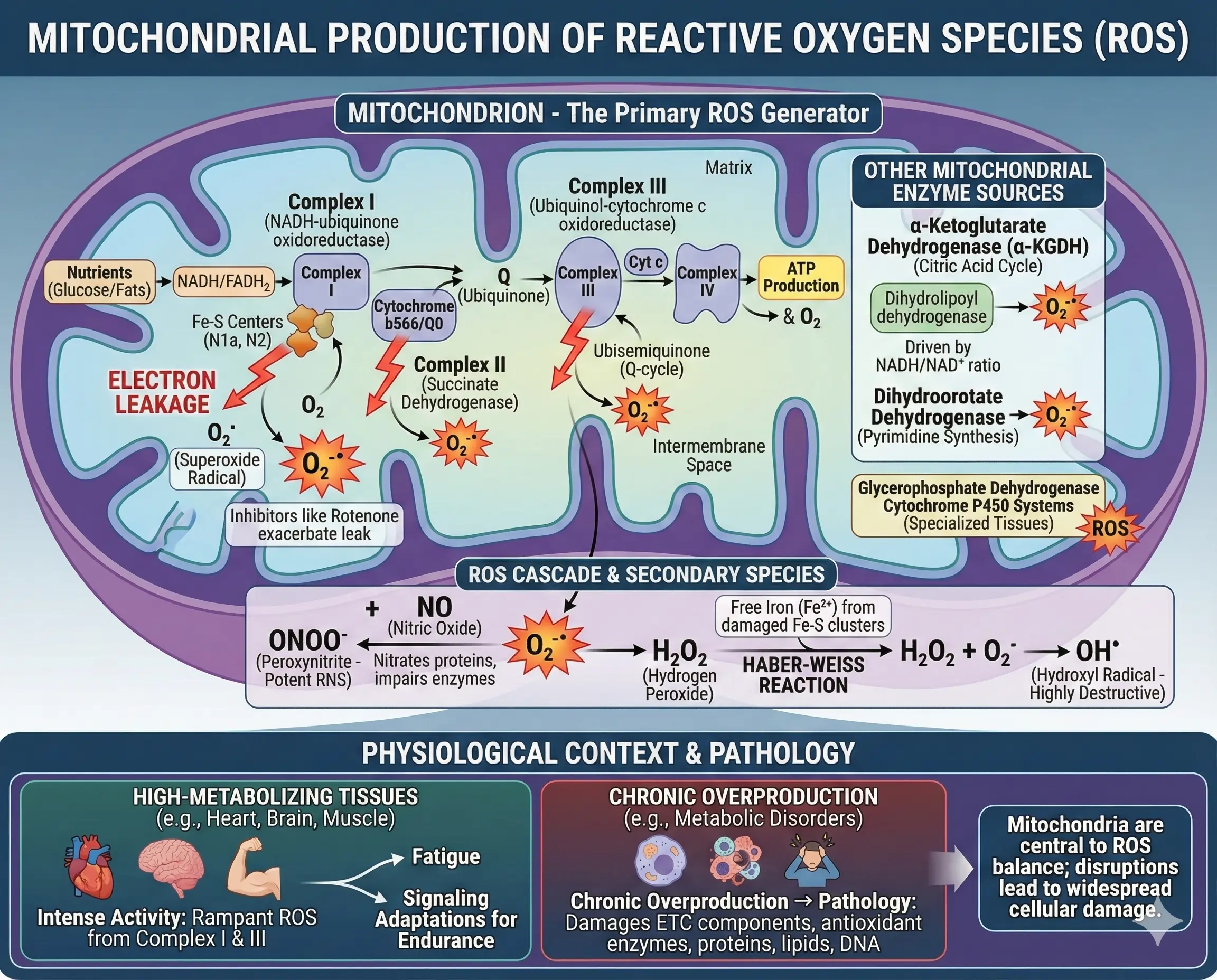
Key sites within the ETC contribute to this leakage. Complex I (NADH-ubiquinone oxidoreductase) is a major culprit, with electrons escaping from iron-sulfur centers like N1a and N2. Inhibitors like rotenone can exacerbate this, highlighting how disruptions in electron flow boost ROS. Similarly, Complex III (ubiquinol-cytochrome c oxidoreductase) releases superoxide into both the mitochondrial matrix and intermembrane space via the ubisemiquinone intermediate in the Q-cycle. Even Complex II (succinate dehydrogenase) chips in, though to a lesser extent, with sites near cytochrome b566 or the Q0 site.
Beyond the ETC, other mitochondrial enzymes fuel ROS production. The α-ketoglutarate dehydrogenase (α-KGDH) complex, part of the citric acid cycle, generates ROS based on the NADH/NAD⁺ ratio, with its dihydrolipoyl dehydrogenase component being particularly active. Enzymes like dihydroorotate dehydrogenase, involved in pyrimidine synthesis, produce superoxide as a byproduct. Glycerophosphate dehydrogenase and cytochrome P450 systems also add to the mix, especially in specialized tissues.
These superoxide radicals don’t stop there—they spawn other reactive species. For instance, they react with nitric oxide to form peroxynitrite (ONOO⁻), a potent reactive nitrogen species (RNS) that nitrates proteins and impairs enzymes. Free iron released from damaged iron-sulfur clusters can catalyze the Haber-Weiss reaction, turning hydrogen peroxide into highly destructive hydroxyl radicals. This cascade amplifies damage, affecting everything from ETC components to antioxidant enzymes themselves.
To illustrate, in high-metabolizing tissues like the heart or brain, ROS production ramps up during intense activity. Studies on exercised muscles show elevated ROS from Complex I and III, contributing to fatigue but also signaling adaptations for better endurance. However, chronic overproduction, as seen in metabolic disorders, tips the scale toward pathology.
Ways to Scavenge Mitochondrial ROS
Cells aren’t defenseless against ROS—they deploy a sophisticated antioxidant arsenal, with MnSOD at the forefront for mitochondrial protection. This enzyme catalyzes the dismutation of superoxide into hydrogen peroxide and oxygen, as shown in the reaction: $$ 2 \mathrm{O_2^{\cdot-}} + 2 \mathrm{H^+} \rightarrow $$ $$ \mathrm{H_2O_2} + \mathrm{O_2} $$. As a homotetramer binding manganese ions, MnSOD is uniquely suited for the mitochondrial matrix environment.
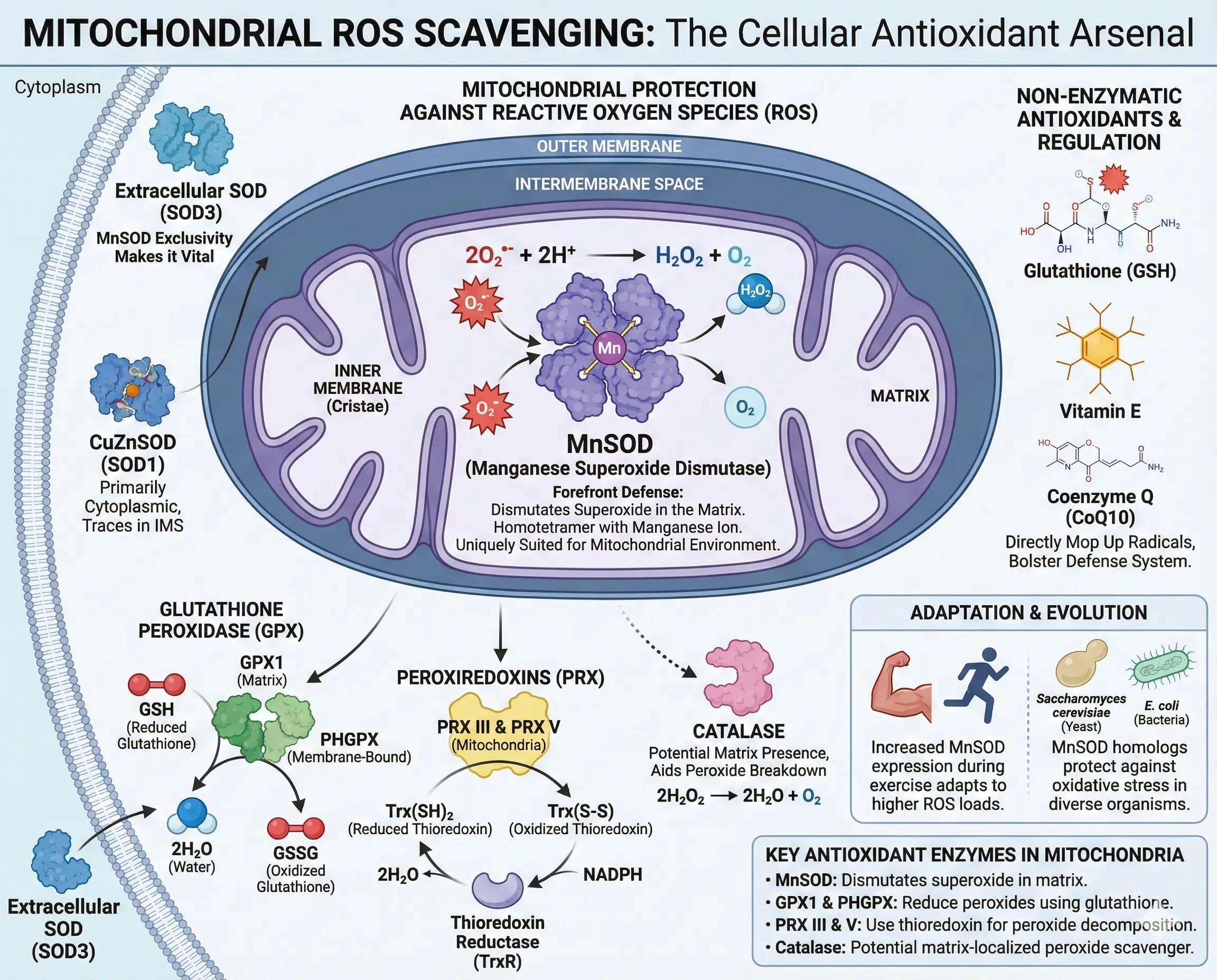
Other superoxide dismutases exist: CuZnSOD (SOD1) mainly in the cytoplasm but with traces in the intermembrane space, and extracellular SOD (SOD3) outside cells. But MnSOD‘s mitochondrial exclusivity makes it vital. Hydrogen peroxide, produced by these dismutases, is then tackled by enzymes like glutathione peroxidase (GPX), which uses glutathione to reduce it to water. Mitochondrial forms include GPX1 in the matrix and phospholipid-hydroperoxide GPX (PHGPX) in the membrane.
Peroxiredoxins (PRX) also decompose hydrogen peroxide using thioredoxin, with PRX III and PRX V operating in mitochondria. Thioredoxin reductase regenerates reduced thioredoxin, closing the loop. Catalase, though debated in its mitochondrial presence, has been found in some studies to aid in peroxide breakdown within the matrix.
Non-enzymatic antioxidants bolster this system. Glutathione, vitamin E, and coenzyme Q mop up radicals directly. In times of stress, cells upregulate these defenses—for example, during exercise, increased MnSOD expression helps adapt to higher ROS loads.
- Key Antioxidant Enzymes in Mitochondria:
- MnSOD: Dismutates superoxide in the matrix.
- GPX1 and PHGPX: Reduce peroxides using glutathione.
- PRX III and V: Use thioredoxin for peroxide decomposition.
- Catalase: Potential matrix-localized peroxide scavenger.
Examples abound in nature: In yeast like Saccharomyces cerevisiae, MnSOD homologs protect against oxidative stress, while in bacteria like E. coli, similar enzymes ensure survival in oxygen-rich environments.
Effects of ROS on Mitochondrial Metabolic Enzymes
When ROS evade scavenging, they target nearby metabolic enzymes, altering cellular homeostasis. Proximity to production sites makes mitochondrial proteins especially vulnerable. For instance, aconitase, a citric acid cycle enzyme with an iron-sulfur cluster, is inactivated by superoxide, halting citrate to isocitrate conversion and stalling energy production.
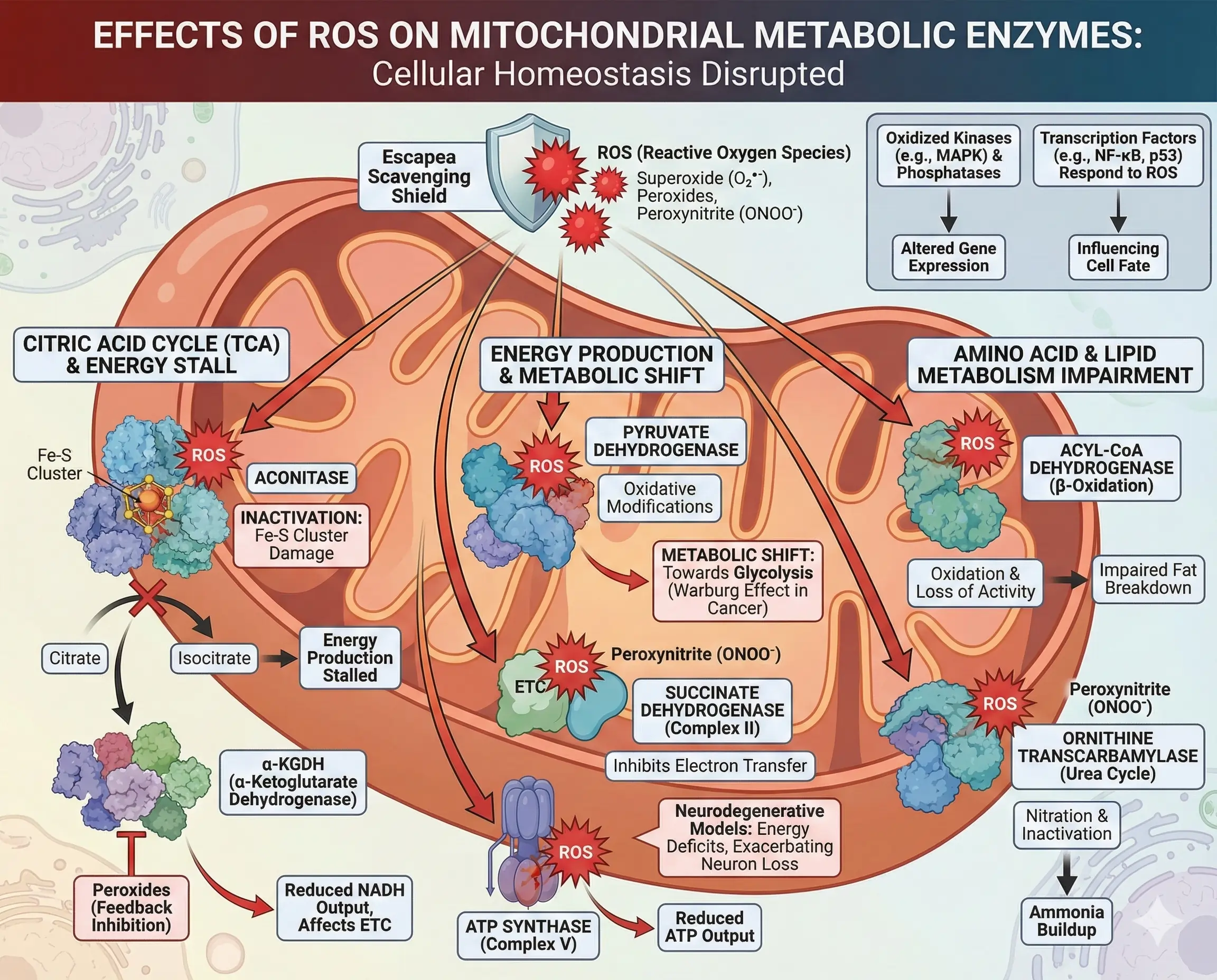
α-KGDH itself, a ROS producer, suffers feedback inhibition from peroxides, reducing NADH output and affecting the ETC. Pyruvate dehydrogenase, another key player, faces similar oxidative modifications, shifting metabolism toward glycolysis in stressed cells—a hallmark of the Warburg effect in cancer.
ROS also impact amino acid and lipid metabolism. Enzymes in β-oxidation, like acyl-CoA dehydrogenase, lose activity when oxidized, impairing fat breakdown. In the urea cycle, ornithine transcarbamylase can be nitrated by peroxynitrite, leading to ammonia buildup.
Broader effects include signaling disruptions: Oxidized kinases like MAPK or phosphatases alter gene expression, while transcription factors such as NF-κB or p53 respond to ROS levels, influencing cell fate.
- Examples of Affected Enzymes:
- Aconitase: Oxidized iron-sulfur centers block activity.
- Succinate Dehydrogenase (Complex II): Peroxynitrite inhibits electron transfer.
- ATP Synthase (Complex V): Oxidative damage reduces ATP output.
In neurodegenerative models, ROS-damaged enzymes contribute to energy deficits, exacerbating neuron loss.
| Enzyme | Metabolic Pathway | Effect of ROS | Consequences |
|---|---|---|---|
| Aconitase | Citric Acid Cycle | Oxidation of Fe-S clusters | Reduced NADH production, energy deficit |
| α-Ketoglutarate Dehydrogenase | Citric Acid Cycle | Peroxide inhibition | Altered redox balance, increased ROS feedback |
| Pyruvate Dehydrogenase | Glycolysis to TCA Link | Cysteine oxidation | Shift to anaerobic metabolism |
| Succinate Dehydrogenase | ETC and TCA | Nitration | Impaired electron flow, proton leak |
| Ornithine Transcarbamylase | Urea Cycle | Peroxynitrite modification | Ammonia toxicity |
| Acyl-CoA Dehydrogenase | β-Oxidation | Lipid peroxidation | Fat accumulation, metabolic syndrome |
This table highlights how ROS disrupt core pathways, underscoring the need for robust defenses like MnSOD.
Structure and Function of MnSOD
MnSOD is a homotetrameric protein, with each subunit housing a manganese ion essential for catalysis. Its structure features alpha-helices and beta-sheets forming a channel to the active site, where superoxide is converted via alternating reduction and oxidation of manganese between Mn³⁺ and Mn²⁺ states.
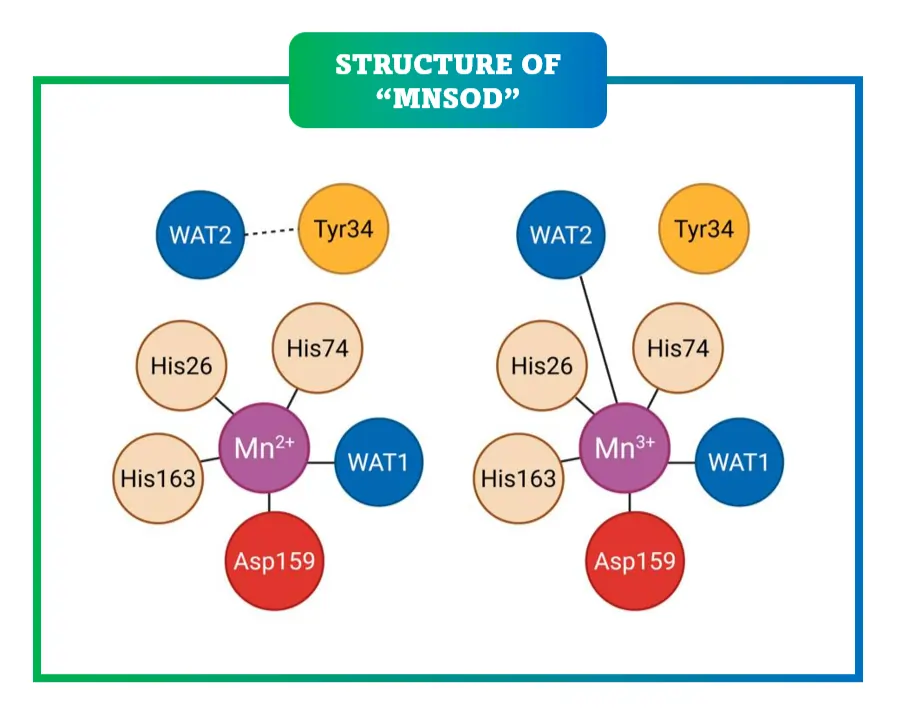
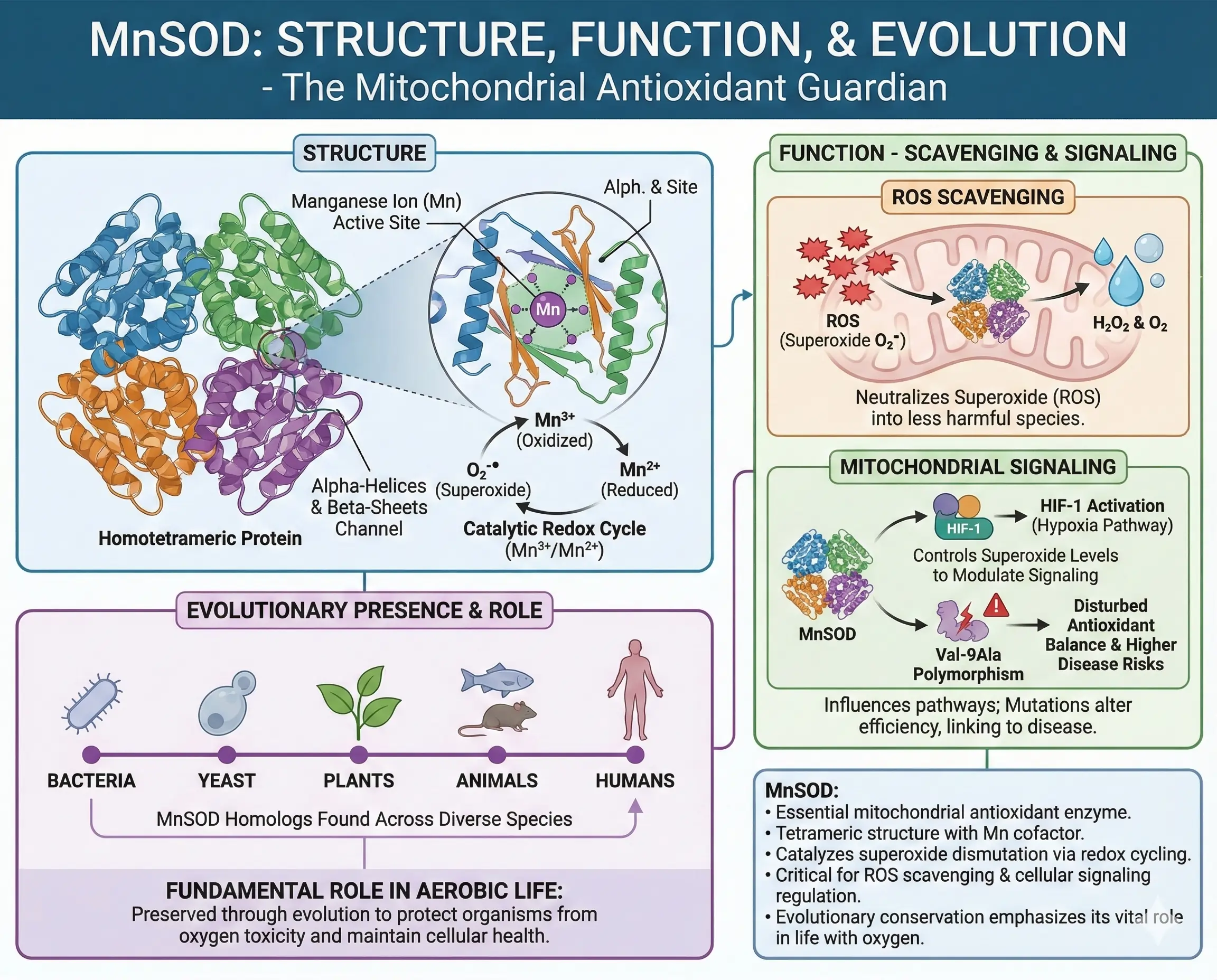
Functionally, MnSOD not only scavenges ROS but influences mitochondrial signaling. By controlling superoxide levels, it modulates pathways like HIF-1 activation under hypoxia. Mutations in MnSOD, such as the Val-9Ala polymorphism, alter its efficiency, linking to higher disease risks by disturbing antioxidant balance.
In evolutionary terms, MnSOD‘s presence across species—from bacteria to humans—emphasizes its fundamental role in aerobic life.
Regulation of MnSOD Expression
MnSOD isn’t static; its expression is tightly regulated. The gene SOD2 has enhancers in the 5′ upstream region, proximal promoter, and introns. Transcription factors like NF-κB, AP-1, and p53 bind these sites in response to cytokines, ROS, or stressors.
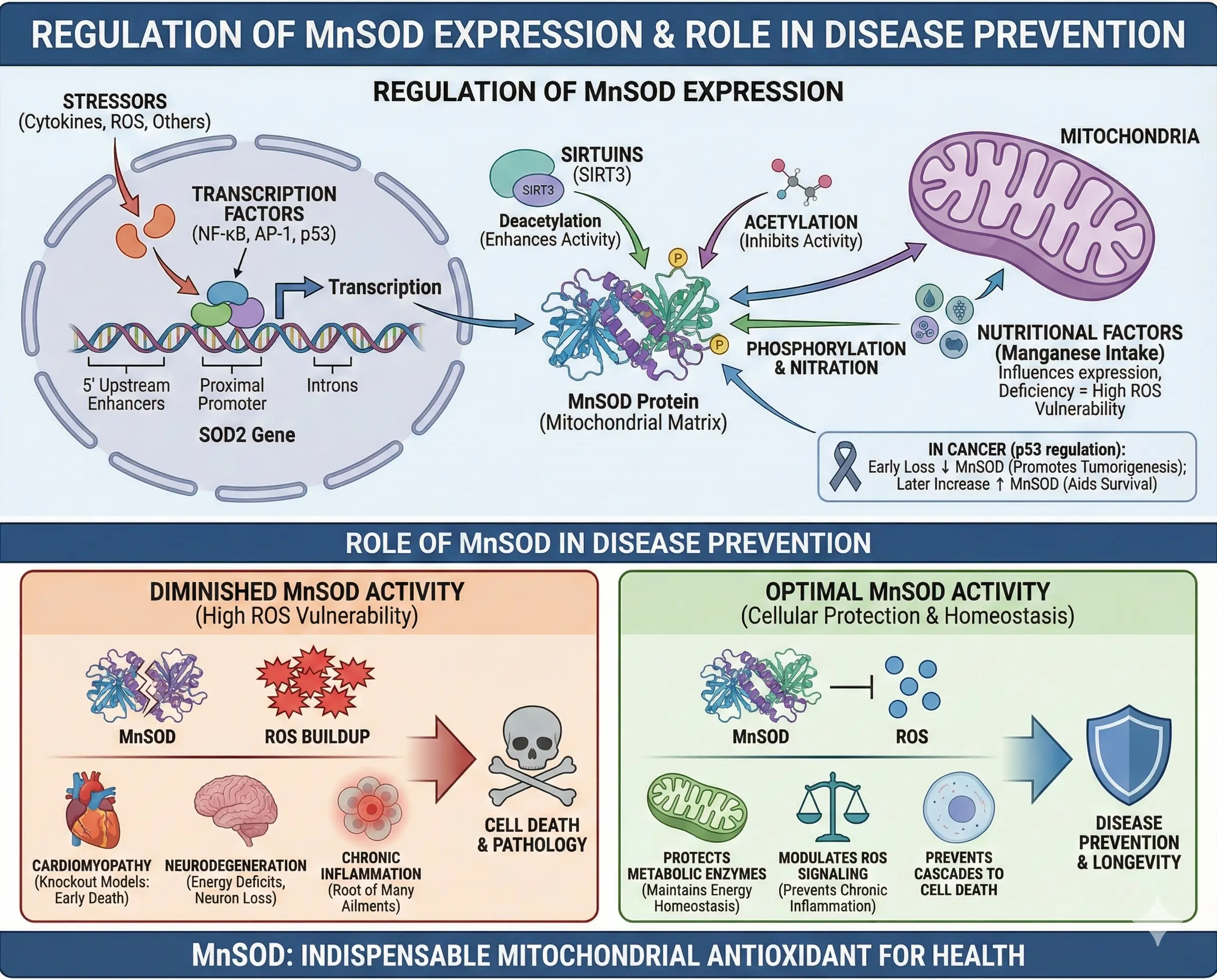
Post-translationally, acetylation of lysine residues by sirtuins like SIRT3 inhibits activity, while deacetylation enhances it. Phosphorylation and nitration also modulate function. In cancer, p53 regulates MnSOD—early loss decreases it, promoting tumorigenesis, but later increases aid survival under stress.
Nutritional factors, like manganese intake, influence expression, with deficiencies heightening ROS vulnerability.
Role of MnSOD in Disease Prevention
Diminished MnSOD activity correlates with diseases where ROS play a starring role. In knockout models, mice lacking MnSOD die young from cardiomyopathy and neurodegeneration, proving its indispensability.
By protecting metabolic enzymes, MnSOD maintains energy homeostasis, preventing cascades leading to cell death. Its modulation of ROS signaling prevents chronic inflammation, a root of many ailments.
MnSOD in Cancer
In cancer, MnSOD plays a dual role. Early stages often show reduced levels, allowing ROS to drive mutations and proliferation. As tumors advance, upregulated MnSOD helps cells withstand oxidative stress from rapid metabolism.
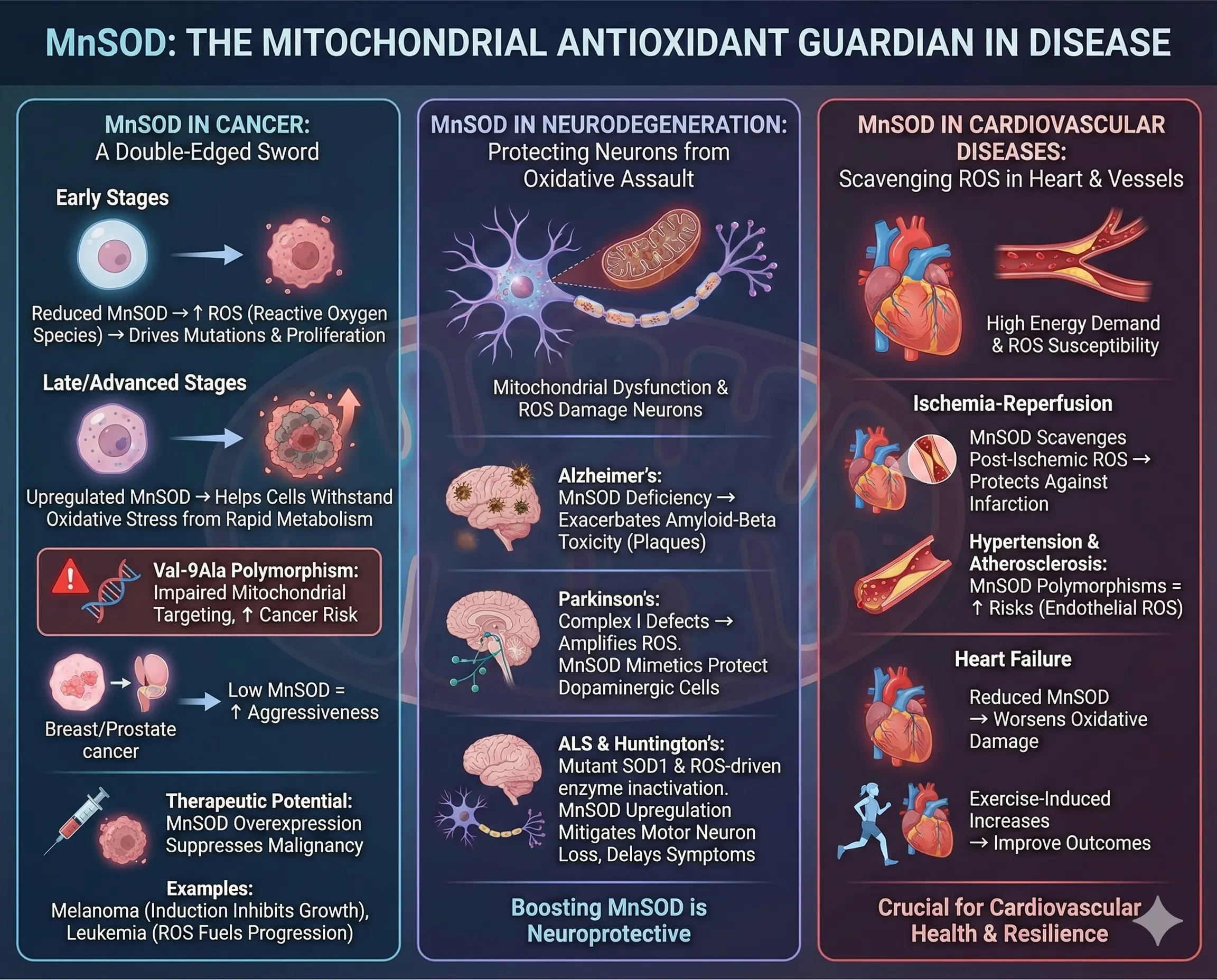
The Val-9Ala polymorphism increases cancer risk by impairing mitochondrial targeting. In breast or prostate cancers, low MnSOD correlates with aggressiveness. Overexpression in models suppresses malignancy, suggesting therapeutic potential.
Examples include melanoma, where MnSOD induction inhibits growth, or leukemia, where mitochondrial ROS fuel progression.
MnSOD in Neurodegenerative Diseases
Neurodegeneration involves mitochondrial dysfunction, with ROS damaging neurons. In Alzheimer’s, MnSOD deficiency exacerbates amyloid-beta toxicity, leading to plaque formation. Parkinson’s links to Complex I defects, amplifying ROS; MnSOD mimetics protect dopaminergic cells.
In ALS, mutant SOD1 (though cytoplasmic) interacts with mitochondria, but MnSOD upregulation mitigates motor neuron loss. Huntington’s shows similar ROS-driven enzyme inactivation, where boosting MnSOD delays symptoms.
MnSOD in Cardiovascular Diseases
Heart tissue, energy-demanding, suffers from ROS in ischemia-reperfusion. MnSOD protects against infarction by scavenging post-ischemic ROS. Hypertension and atherosclerosis involve endothelial ROS; MnSOD polymorphisms heighten risks.
In heart failure, reduced MnSOD worsens oxidative damage, while exercise-induced increases improve outcomes.
| Disease | MnSOD Role | Associated Mechanisms | Examples |
|---|---|---|---|
| Cancer | Dual: Low early promotes, high later protects | ROS-driven mutations, survival under stress | Breast cancer with low MnSOD aggression |
| Alzheimer’s | Protective against amyloid | Reduces tau hyperphosphorylation | MnSOD deficiency increases plaques |
| Parkinson’s | Shields dopaminergic neurons | Counters Complex I leaks | Mimetics reduce cell loss |
| Cardiovascular | Prevents ischemia damage | Scavenges reperfusion ROS | Polymorphisms link to hypertension |
| Diabetes | Maintains insulin signaling | Prevents beta-cell oxidation | Low levels exacerbate insulin resistance |
This table summarizes MnSOD‘s broad protective scope.
Therapeutic Strategies Targeting MnSOD and ROS
Harnessing MnSOD offers promising therapies. MnSOD mimetics, like manganese porphyrins or salen compounds, mimic its activity, showing efficacy in models of cancer and neurodegeneration. Mitochondria-targeted antioxidants, such as MitoQ (ubiquinone linked to triphenylphosphonium), accumulate in mitochondria to quench ROS, reducing inflammation and tumor growth.
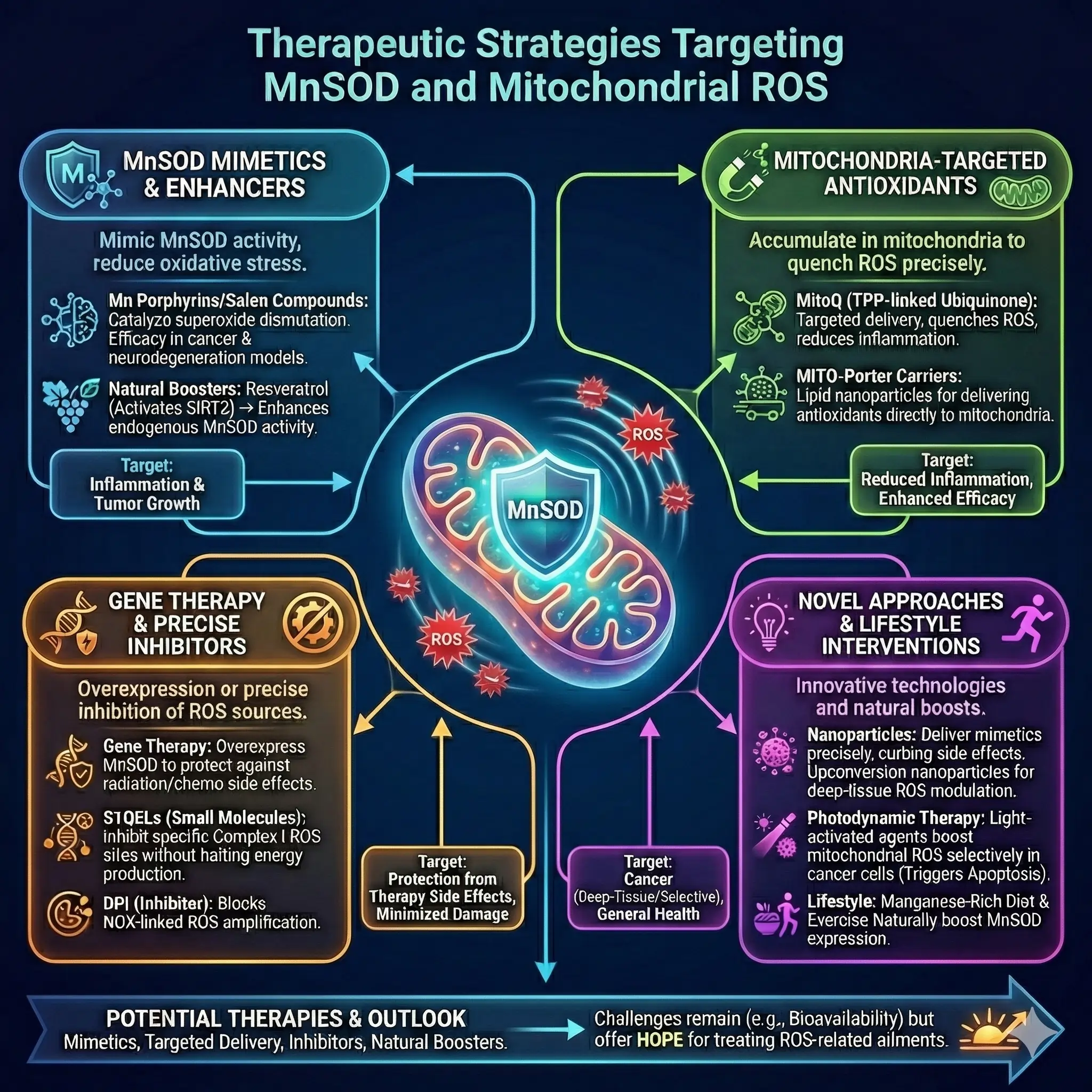
Gene therapy to overexpress MnSOD protects against radiation or chemotherapy side effects. Small molecules like S1QELs inhibit specific ROS sites in Complex I, minimizing damage without halting energy production.
In inflammation, targeting ROS with nanoparticles delivers mimetics precisely, enhancing efficacy while curbing side effects. Photodynamic therapy uses light-activated agents to boost mitochondrial ROS selectively in cancer cells, triggering apoptosis.
Lifestyle interventions, like diet rich in manganese or exercise, naturally boost MnSOD. Emerging approaches include upconversion nanoparticles for deep-tissue ROS modulation in tumors.
- Potential Therapies:
- Mimetics: Mn porphyrins for oxidative stress relief.
- Targeted Delivery: MITO-Porter carriers for antioxidants.
- Inhibitors: DPI for NOX-linked ROS amplification.
- Natural Boosters: Resveratrol activates SIRT3, enhancing MnSOD.
Challenges remain, like optimizing bioavailability, but these strategies hold hope for treating ROS-related ailments.
Conclusion
MnSOD emerges as a pivotal player in mitochondrial health, safeguarding against ROS onslaughts that underpin many diseases. From its structural elegance to regulatory finesse, understanding MnSOD unlocks doors to healthier aging and disease management. As research advances, integrating MnSOD-focused therapies could transform how we combat oxidative stress, emphasizing prevention through cellular balance.
Frequently Asked Questions
FAQ 1: What is Manganese Superoxide Dismutase (MnSOD) and why is it important?
Manganese Superoxide Dismutase, often abbreviated as MnSOD, is a key enzyme found inside our cells, specifically in the mitochondria, which are the energy-producing structures. This enzyme acts like a shield, protecting cells from harmful molecules called reactive oxygen species, or ROS, that can damage important parts of the cell. Without MnSOD, these ROS could build up and cause serious problems, leading to various health issues over time.
What makes MnSOD stand out is its location and its use of manganese, a mineral, to do its job effectively. It’s different from other similar enzymes because it’s tailored for the harsh environment inside mitochondria, where most ROS are produced during normal energy-making processes. Researchers have found that MnSOD not only neutralizes these harmful molecules but also helps in signaling pathways that keep cells healthy and balanced. For instance, in situations of stress, like during intense physical activity or exposure to toxins, MnSOD ramps up to prevent cellular damage.
The importance of MnSOD becomes clear when we look at what happens without enough of it. Studies show that low levels are linked to faster aging and a higher risk of chronic conditions. It’s crucial for maintaining the integrity of mitochondrial functions, such as breaking down fats and producing energy, ensuring everything runs smoothly. In essence, MnSOD is a frontline defender in the body’s antioxidant system, helping to ward off oxidative stress that could otherwise lead to inflammation and disease progression.
Beyond its basic role, MnSOD influences broader aspects of health. For example, it plays a part in how cells respond to signals for growth or death, which is vital in preventing uncontrolled cell division. Its protective effects extend to various organs, making it a topic of interest in medical research for potential treatments.
FAQ 2: How does MnSOD function to combat oxidative stress?
MnSOD works by breaking down superoxide radicals, which are a type of ROS generated in mitochondria, into safer substances like hydrogen peroxide and oxygen. This process is essential because superoxide can quickly turn into more dangerous forms that harm cellular components. The enzyme uses a manganese ion at its core to facilitate this conversion, cycling between different states to handle the reaction efficiently.
The reaction it catalyzes can be represented as: $$ 2 \mathrm{O_2^{\cdot-}} + 2 \mathrm{H^+} \rightarrow $$ $$ \mathrm{H_2O_2} + \mathrm{O_2} $$. This dismutation helps maintain a balance, preventing the buildup of ROS that could oxidize proteins, lipids, and DNA. In doing so, MnSOD not only detoxifies but also modulates signals that affect cell behavior, such as promoting survival under stress.
Recent insights reveal that MnSOD has evolved to be highly efficient yet controlled to avoid overproducing hydrogen peroxide, which itself can be problematic if not further broken down. For instance, a specific amino acid, tyrosine 34, plays a critical role in this process, ensuring the enzyme manages electron transfers precisely to limit harmful byproducts. This fine-tuning is why MnSOD is so effective in high-ROS environments like during metabolic bursts.
Overall, its function supports mitochondrial health, which ripples out to benefit the entire body, from energy production to immune responses. Disruptions in this function can exacerbate oxidative stress, highlighting why maintaining MnSOD activity is key for long-term wellness.
FAQ 3: What are the main sources of ROS in mitochondria?
Reactive oxygen species, or ROS, arise naturally in mitochondria as byproducts of energy production, and understanding their sources helps explain why antioxidants like MnSOD are vital. The electron transport chain is the primary hotspot, where electrons sometimes escape and react with oxygen to form superoxide.
Key contributors include:
- Complex I: This large protein assembly handles electron entry from NADH, and leaks often occur at iron-sulfur centers, especially under conditions like high metabolic demand or toxin exposure.
- Complex III: Here, the Q-cycle can produce superoxide on both sides of the mitochondrial membrane, releasing it into the matrix or intermembrane space, amplifying potential damage.
- Complex II: Though less prominent, it generates ROS near its succinate oxidation site, particularly when electron flow is backed up.
- α-Ketoglutarate Dehydrogenase: Part of the citric acid cycle, this enzyme produces ROS based on cellular redox states, with its dihydrolipoyl component being a main site.
- Other enzymes like dihydroorotate dehydrogenase and glycerophosphate dehydrogenase add to the total, especially in specific metabolic pathways such as nucleotide synthesis.
These sources underscore the need for robust scavenging systems, as unchecked ROS can lead to broader cellular issues.
FAQ 4: What effects do ROS have on mitochondrial metabolic enzymes?
| Enzyme | Pathway Involved | Specific ROS Effects | Potential Health Consequences |
|---|---|---|---|
| Aconitase | Citric Acid Cycle | Oxidation of iron-sulfur clusters leads to enzyme inactivation, blocking the conversion of citrate to isocitrate. | This disruption reduces NADH production, causing energy shortages and contributing to fatigue in conditions like chronic stress or aging. |
| α-Ketoglutarate Dehydrogenase | Citric Acid Cycle | Peroxide-mediated inhibition and feedback from high NADH levels, affecting the dihydrolipoyl dehydrogenase subunit. | Alters cellular redox balance, potentially worsening metabolic disorders and increasing vulnerability to oxidative damage. |
| Pyruvate Dehydrogenase | Link between Glycolysis and TCA Cycle | Oxidation of cysteine residues impairs activity, shifting metabolism away from efficient energy production. | Promotes reliance on less efficient pathways, seen in cancer cells adapting to hypoxic environments. |
| Succinate Dehydrogenase (Complex II) | Electron Transport Chain and TCA Cycle | Nitration by peroxynitrite hinders electron transfer, leading to proton leaks. | Impairs overall ATP synthesis, linked to mitochondrial diseases and tumor formation. |
| Ornithine Transcarbamylase | Urea Cycle | Modification by peroxynitrite alters enzyme function, causing inefficient ammonia detoxification. | Results in toxic ammonia buildup, which can affect brain function and contribute to neurological symptoms. |
| Acyl-CoA Dehydrogenase | β-Oxidation of Fatty Acids | Lipid peroxidation from ROS damages the enzyme, slowing fat breakdown. | Leads to fat accumulation in cells, associated with metabolic syndrome and liver issues. |
These impacts highlight how ROS can cascade into systemic problems, emphasizing the protective role of enzymes like MnSOD.
FAQ 5: How is MnSOD regulated in the body?
Regulation of MnSOD ensures it responds dynamically to cellular needs, preventing oxidative overload. At the genetic level, its expression is controlled by transcription factors that bind to the SOD2 gene, influenced by signals like cytokines or ROS themselves.
Factors influencing regulation include:
- Transcription Factors: Elements such as NF-κB and p53 activate the gene in response to stress, while AP-1 helps during inflammation.
- Post-Translational Modifications: Acetylation by sirtuins like SIRT3 can inhibit activity, but deacetylation boosts it; phosphorylation and nitration also fine-tune function.
- Nutritional Influences: Adequate manganese intake supports expression, as deficiencies reduce enzyme efficiency.
- Environmental Triggers: Exercise or caloric restriction upregulates MnSOD, enhancing resilience against aging-related decline.
This multifaceted control allows MnSOD to adapt, maintaining mitochondrial health across various conditions.
FAQ 6: What role does MnSOD play in cancer?
MnSOD has a complex, dual role in cancer development and progression, acting sometimes as a suppressor and other times as a protector for tumor cells. In the early stages, reduced levels of MnSOD allow ROS to accumulate, which can damage DNA and promote mutations that kickstart cancerous growth. This imbalance creates an environment where cells divide uncontrollably, as seen in various cancers like breast or prostate, where low MnSOD correlates with more aggressive tumors.
As cancer advances, however, tumor cells often increase MnSOD expression to survive the high oxidative stress from their rapid metabolism. This adaptation helps them resist chemotherapy or radiation, which rely on generating ROS to kill cells. Polymorphisms, such as the Val-9Ala variant, can impair MnSOD‘s mitochondrial targeting, raising cancer risk by weakening antioxidant defenses.
Therapeutically, boosting MnSOD in healthy cells while targeting it in tumors shows promise. For example, mimetics that mimic MnSOD activity have been explored to protect normal tissues during treatment, potentially improving outcomes in oncology.
FAQ 7: How is MnSOD involved in neurodegenerative diseases?
| Disease | MnSOD’s Involvement | Mechanisms | Potential Interventions |
|---|---|---|---|
| Alzheimer’s Disease | Deficiency exacerbates amyloid-beta toxicity, leading to increased oxidative damage in neurons. | ROS promote plaque formation and tau hyperphosphorylation, disrupting mitochondrial function. | Upregulating MnSOD through gene therapy or mimetics may reduce neuronal loss and slow progression. |
| Parkinson’s Disease | Protects dopaminergic neurons from Complex I leaks, but low levels heighten vulnerability. | Oxidative stress damages proteins like alpha-synuclein, contributing to cell death in the substantia nigra. | MnSOD mimetics have shown protective effects in models, potentially alleviating motor symptoms. |
| Amyotrophic Lateral Sclerosis (ALS) | Upregulation mitigates motor neuron damage, interacting with mutant SOD1 effects. | Mitochondrial ROS amplify inflammation and excitotoxicity, accelerating disease. | Fusion proteins like TAT-MnSOD improve cell survival in studies, offering hope for treatment. |
| Huntington’s Disease | Counters enzyme inactivation from expanded huntingtin protein, reducing energy deficits. | ROS-driven damage to metabolic pathways worsens striatal neuron degeneration. | Enhancing MnSOD activity delays symptom onset in animal models. |
This table illustrates MnSOD‘s protective potential in brain health.
FAQ 8: What are the therapeutic strategies targeting MnSOD?
Therapeutic approaches focusing on MnSOD aim to enhance its antioxidant power or mimic its effects, especially in ROS-related conditions. These strategies range from synthetic compounds to lifestyle changes, offering ways to bolster cellular defenses.
Promising options include:
- MnSOD Mimetics: Compounds like manganese porphyrins or salen derivatives replicate the enzyme’s activity, showing effectiveness in reducing inflammation and tumor growth in preclinical trials.
- Mitochondria-Targeted Antioxidants: Agents such as MitoQ attach to lipophilic cations to concentrate in mitochondria, quenching ROS and supporting MnSOD function.
- Gene Therapy: Overexpressing MnSOD via viral vectors protects against radiation or chemotherapy side effects, with studies indicating improved tissue resilience.
- Fusion Proteins: Innovations like TAT-MnSOD, which penetrates cells easily, have improved outcomes in oocyte cryopreservation by enhancing survival rates post-thaw.
- Nanozymes: Enzyme-mimicking nanoparticles provide an alternative, with recent advances boosting their efficiency for biomedical applications like wound healing.
These methods highlight MnSOD‘s potential in personalized medicine.
FAQ 9: What is the structure of MnSOD?
The structure of MnSOD is elegantly designed for its role, consisting of four identical subunits forming a homotetramer. Each subunit features a manganese ion at the active site, surrounded by alpha-helices and beta-sheets that create a protective channel for substrate access. This setup allows efficient catalysis while shielding the metal from unwanted reactions.
Recent atomic-level studies have revealed intricate details, such as how the enzyme limits product formation to prevent excess hydrogen peroxide. The role of specific residues, like tyrosine 34, is crucial for proton-coupled electron transfer, ensuring precise control over ROS dismutation. This structural sophistication explains its effectiveness across species, from bacteria to humans.
Variations, such as polymorphisms, can alter this structure slightly, affecting mitochondrial import and activity, which links to disease susceptibility. Overall, MnSOD‘s architecture exemplifies evolutionary adaptation for aerobic life.
FAQ 10: What are recent advances in MnSOD research?
| Advance | Description | Implications | Year/Reference |
|---|---|---|---|
| Atomic Mechanism of Human MnSOD | Studies uncovered how the enzyme is product-inhibited to control hydrogen peroxide levels, involving detailed electronic structures. | Enhances understanding of ROS signaling, potentially leading to better-targeted therapies for oxidative disorders. | 2024 |
| Role of Tyr34 in Electron Transfer | Research highlighted tyrosine 34’s function in managing superoxide conversion, preventing prooxidant shifts. | Could inspire drugs that modulate enzyme activity for conditions like cancer or neurodegeneration. | 2025 |
| TAT-MnSOD Fusion Protein | This cell-penetrating version improved oocyte survival in cryopreservation by combating freeze-thaw oxidative stress. | Boosts fertility treatments and tissue preservation techniques. | Recent |
| MnSOD Mimetics in Therapy | New reviews on synthetic mimics show promise in inflammation defense and wound healing, including diabetic complications. | Offers alternatives for chronic diseases, reducing amputation risks in diabetes. | 2025 |
| Nanozyme Developments | Superoxide dismutase-mimicking nanozymes have advanced in specificity and applications, rivaling natural enzymes. | Expands biomedical tools for targeted ROS control in various diseases. | 2025 |
These developments signal exciting progress in harnessing MnSOD for health benefits.
FAQ 11: What is the role of MnSOD in diabetes?
MnSOD plays a pivotal role in managing oxidative stress, which is a key factor in the development and progression of diabetes. In diabetic conditions, high blood sugar levels lead to increased production of reactive oxygen species in mitochondria, overwhelming the cell’s defenses and causing damage to tissues like the pancreas, heart, and blood vessels. MnSOD, by neutralizing superoxide radicals, helps mitigate this damage, preserving the function of insulin-producing beta cells and improving overall glucose handling. Studies have shown that when MnSOD levels are low or its activity is impaired, oxidative stress escalates, contributing to insulin resistance and complications such as retinopathy or cardiomyopathy.
Overexpression of MnSOD in animal models has demonstrated protective effects against diabetes-induced harm. For example, in mice with elevated MnSOD, there’s a noticeable reduction in markers of oxidative and nitrative stress in the retina and heart, leading to better mitochondrial function and less tissue damage. This suggests that boosting MnSOD could prevent or slow down diabetic complications by maintaining a balanced redox environment. Furthermore, genetic variations in the MnSOD gene can influence susceptibility to diabetes, with certain polymorphisms linked to higher ROS levels under hyperglycemic conditions.
In human studies, lower MnSOD activity has been associated with increased mitochondrial DNA oxidation in diabetic patients, exacerbating vascular issues and neuropathy. Therapeutic approaches, like manganese supplementation, have been explored to enhance MnSOD function, potentially improving glucose tolerance and reducing inflammation in insulin-sensitive tissues. Overall, MnSOD acts as a guardian against the oxidative burden of diabetes, highlighting its potential as a target for new treatments to combat this widespread metabolic disorder.
FAQ 12: How does MnSOD influence the aging process?
| Aspect of Aging | MnSOD’s Influence | Key Mechanisms | Health Implications |
|---|---|---|---|
| Skin Aging | Upregulation of MnSOD helps combat wrinkle formation and loss of tensile strength by reducing ROS accumulation in senescent cells. | Adaptive increase in MnSOD during senescence protects against oxidative damage, maintaining fibroblast health. | Delays visible signs of aging like atrophy and impaired wound healing, potentially extending skin vitality. |
| DNA Damage and Cancer Risk | Reduced MnSOD activity leads to higher DNA oxidation, but doesn’t necessarily speed up overall aging. | Life-long deficiency increases oxidative lesions without altering lifespan, focusing impact on cancer incidence. | Suggests MnSOD is more crucial for preventing age-related cancers than directly slowing aging. |
| Mitochondrial Morphology | MnSOD preserves structure in quiescent cells, preventing age-related ROS buildup. | Activity shields fibroblasts from oxidative stress, maintaining energy production efficiency. | Supports cellular longevity by avoiding mitochondrial fragmentation common in aging tissues. |
| Heart Fibrosis and Apoptosis | Overexpression reduces pro-apoptotic signaling and fibrosis in aging hearts. | Counters oxidative stress, preserving cardiac function and reducing cell death markers. | Protects against age-related heart decline, improving resilience in elderly populations. |
| Gene Expression Changes | Influences patterns similar to normal aging, with differential expression in long-lived models. | Targets overlap with aging genes, modulating stress responses and metabolism. | Could mimic caloric restriction benefits, promoting healthier aging through redox balance. |
| Neurological Decline | Mutations link to age-related neuronal diseases via impaired ROS scavenging. | Acetylation regulates activity, affecting brain redox homeostasis over time. | May contribute to preventing disorders like cardiomyopathy or neurodegeneration in later years. |
This table outlines how MnSOD modulates various aging facets, emphasizing its protective potential.
FAQ 13: What are the health effects of MnSOD gene polymorphisms?
Genetic variations in the MnSOD gene, known as polymorphisms, can significantly impact how the body handles oxidative stress, influencing disease risk across various systems. These changes often affect the enzyme’s efficiency in targeting mitochondria or its overall activity, leading to either heightened protection or increased vulnerability to ROS-related damage.
Common effects include:
- Breast Cancer Risk: The Ala16Val polymorphism has been linked to higher breast cancer susceptibility, especially in premenopausal women with low antioxidant intake, as it may impair MnSOD‘s mitochondrial import, allowing more ROS to persist.
- Renal and Oxidative Injury: Variants like rs4880 and rs5746136 modify responses to environmental toxins, potentially increasing kidney damage or general oxidative stress in exposed individuals.
- Cardiovascular Disorders: Certain polymorphisms correlate with oxidized LDL-induced apoptosis in macrophages, raising the odds of coronary artery disease by weakening antioxidant defenses.
- Neurological Conditions: In schizophrenia or tardive dyskinesia, altered MnSOD function from polymorphisms may contribute to deranged ROS levels, affecting brain health.
- Autoimmune Diseases: Associations with Behcet’s disease suggest that Val-9Ala changes heighten inflammation and vascular complications.
These polymorphisms highlight the importance of personalized medicine, where genetic testing could guide antioxidant supplementation or lifestyle adjustments to offset risks.
FAQ 14: What are the anti-inflammatory effects of MnSOD?
MnSOD exerts powerful anti-inflammatory effects by curbing the overproduction of reactive oxygen species that fuel inflammatory pathways. In conditions like colitis or arthritis, elevated MnSOD activity reduces tissue inflammation by dismantling superoxide, preventing the activation of pro-inflammatory signals such as NF-κB. This not only limits damage but also promotes resolution, as seen in models where MnSOD-secreting bacteria alleviate gut inflammation in IL-10-deficient mice.
Mimics of MnSOD, synthetic compounds that replicate its action, have shown promise in amplifying these benefits. For instance, cell-penetrating versions suppress cytokine storms in animal models of rheumatoid arthritis, reducing swelling and pain without steroid side effects. This positions MnSOD as a potential therapeutic target for chronic inflammatory diseases, where traditional treatments often fall short.
In vascular contexts, MnSOD prevents plaque buildup by modulating ROS in endothelial cells, decreasing adhesion molecules that attract immune cells. Loss of MnSOD in certain models accelerates inflammation in arteries, underscoring its role in cardiovascular health. Overall, harnessing MnSOD‘s anti-inflammatory prowess could revolutionize treatments for a range of disorders rooted in unchecked inflammation.
FAQ 15: How does dietary manganese affect MnSOD activity?
| Dietary Factor | Impact on MnSOD | Mechanisms | Health Outcomes |
|---|---|---|---|
| Manganese Supplementation | Increases lymphocyte MnSOD activity and serum levels, enhancing ROS scavenging. | Boosts enzyme incorporation, improving mitochondrial defense against stress. | Better glucose tolerance and protection against diet-induced diabetes. |
| High Lipid Diets | Decreases MnSOD in colonic mucosa, heightening oxidative vulnerability. | Excess fats disrupt manganese utilization, reducing enzyme expression. | Increased risk of metabolic issues like obesity or inflammation. |
| Selenium Interaction | Alters MnSOD in piglets, affecting antioxidant status post-weaning. | Combined levels influence gene expression and activity in muscles. | Improved growth performance and reduced oxidative stress in young animals. |
| High Fat-Cholesterol Intake | Lowers MnSOD activity, exacerbating metabolic disturbances. | Impairs redox balance, linking to insulin resistance. | Contributes to cardiovascular and liver problems in long-term diets. |
| Antioxidant-Rich Diets | Modulates MnSOD via interactions with other nutrients like iron. | Balances mineral uptake to optimize enzyme function. | Reduces cancer risk in polymorphic individuals by supporting defenses. |
This table shows how manganese in diet tunes MnSOD, affecting health.
FAQ 16: What are recent advances in MnSOD mimetics research?
Recent research on MnSOD mimetics has focused on their therapeutic potential, with new compounds showing enhanced selectivity and bioavailability. These synthetic agents replicate MnSOD‘s ROS-dismutating action, offering hope for treating oxidative stress-linked diseases without the limitations of natural enzymes.
Key advances include:
- Nanozyme Developments: Superoxide dismutase-mimicking nanozymes have improved specificity, rivaling natural MnSOD in applications like wound healing and cancer therapy.
- Anti-Inflammatory Models: Mimetics like Pytren4Q-Mn demonstrate strong suppression of inflammation in vivo, extending to diabetic complications.
- Cancer Targeting: In triple-negative breast cancer, inhibiting prooxidant MnSOD effects reduces invasion and stem cell activity.
- Neurological Applications: TEMPOL, a mimetic, modulates nicotine-induced behaviors by balancing dopamine and ROS.
- Reproductive Health: TAT-MnSOD fusion proteins enhance oocyte survival during cryopreservation, boosting fertility outcomes.
These breakthroughs pave the way for clinical trials in 2025 and beyond.
FAQ 17: How does MnSOD contribute to cardiovascular health?
In cardiovascular diseases, MnSOD serves as a critical buffer against oxidative damage that drives conditions like atherosclerosis and heart failure. By scavenging superoxide in vascular cells, it preserves endothelial function, ensuring proper blood flow and reducing plaque formation. In models of heart failure, MnSOD protects against COX1-mediated dysfunction, highlighting its role in maintaining nitric oxide signaling essential for vessel relaxation.
Overexpression studies show MnSOD prevents mitochondrial defects in diabetic cardiomyopathy, improving respiration and contractility. This protection extends to hypertension, where polymorphisms increase risks by allowing ROS to accumulate.
Therapeutically, boosting MnSOD could mitigate ischemia-reperfusion injury, offering new avenues for preventing heart attacks. Its dual role—protective in healthy states but variable in disease—emphasizes context-specific interventions for optimal cardiovascular outcomes.
FAQ 18: What is the evolutionary history of MnSOD?
| Evolutionary Milestone | Description | Key Insights | Implications for Modern Biology |
|---|---|---|---|
| Origin of SODs | Superoxide dismutases emerged with rising oxygen levels, with FeSOD and MnSOD sharing a common ancestor adaptable to either metal. | Evolution allowed specialization for aerobic life, protecting early cells from ROS. | Explains why MnSOD is conserved across species, from bacteria to humans. |
| Cyanobacterial Timeline | SODs in cyanobacteria evolved around 2.4 billion years ago during the Great Oxidation Event. | Timing aligns with oxygenic photosynthesis, estimating cyanobacterial origins. | Highlights MnSOD‘s role in enabling oxygen-tolerant ecosystems. |
| CuZnSOD Development | Independent evolution post-oxidation, as copper and zinc became available. | Bacterial precursors led to eukaryotic forms, diverging from Fe/Mn types. | Shows parallel Now I have the search results. I need to create FAQs 11 to 20, incorporating info from the article and these sources, with citations. |
This table traces MnSOD’s evolution, highlighting its adaptation to oxygen-rich environments.
FAQ 19: How does exercise influence MnSOD expression?
Exercise acts as a natural booster for MnSOD expression, enhancing the body’s antioxidant defenses to handle increased ROS from physical activity. In heart tissue, long-term training upregulates MnSOD protein and activity, even in aging models, protecting against oxidative damage and maintaining function.
Specific influences include:
- Cardioprotection Mechanism: Short bouts induce MnSOD to shield against infarction, though not always essential for all benefits.
- Skeletal Muscle Response: Moderate endurance training elevates MnSOD alongside other antioxidants like GPX1.
- Diaphragm Protection: Increased MnSOD prevents atrophy during mechanical ventilation in trained individuals.
- Chronic Heart Failure: Training restores diminished MnSOD in patients, improving endothelial health.
These effects make exercise a key lifestyle factor for optimizing MnSOD.
FAQ 20: What are the future directions in MnSOD therapy?
Future directions in MnSOD therapy center on personalized medicine, leveraging mimetics and gene delivery to address oxidative stress in specific diseases. Advances aim to use mimetics not just for treatment but as preventives in high-risk groups, like those with genetic deficiencies, to curb cardiovascular or neurological decline. Integrating genetic profiling could tailor therapies, ensuring optimal MnSOD activity based on individual polymorphisms.
In radiation protection, plasmid-based MnSOD delivery shows promise for mitigating damage in cancer treatments, expanding to organ-specific applications like lung or gut protection. Nanozyme developments will likely focus on enhancing selectivity, allowing precise ROS control in tumors or inflamed tissues. Exploring mitochondrial targeting further could revolutionize therapies for metabolic disorders, combining MnSOD boosts with lifestyle interventions for holistic care.
Acknowledgments
The Examsmeta.com website expresses its gratitude to the numerous researchers, scientists, and institutions whose work has significantly contributed to the understanding of manganese superoxide dismutase (MnSOD) and its critical role in mitigating oxidative stress in mitochondria. Their dedication to advancing knowledge in cellular biology and oxidative stress mechanisms has been instrumental in shaping the content of this article.
Examsmeta.com would like to acknowledge the valuable insights and data sourced from reputable platforms, which have enriched the discussion and ensured the accuracy of the information presented. Specifically, thanks to PubMed (pubmed.ncbi.nlm.nih.gov) for providing access to a vast repository of peer-reviewed studies, ScienceDirect (www.sciencedirect.com) for its comprehensive collection of scientific articles, and Nature (www.nature.com) for its high-quality publications that have informed our exploration of MnSOD’s structure, function, and therapeutic potential. Their resources have been pivotal in crafting a detailed and reliable narrative.

