NADPH oxidases, often abbreviated as NOXs, are a fascinating family of enzymes that play a crucial role in our body’s production of reactive oxygen species, or ROS. These molecules are like double-edged swords: they help with cell signaling and defense against pathogens, but when overproduced, they can lead to oxidative stress and contribute to serious health issues.
In this in-depth article, we’ll explore everything from the basic molecular mechanisms of NOXs to the latest inhibitors being studied for therapeutic use. Whether you’re a student, researcher, or just curious about biology, this guide breaks it down in straightforward terms, drawing from key scientific insights.
. 2023 Aug 31;66(17):11632–11655. doi: 10.1021/acs.jmedchem.3c00770)
Table of Contents
What Are Reactive Oxygen Species and Why Do They Matter?
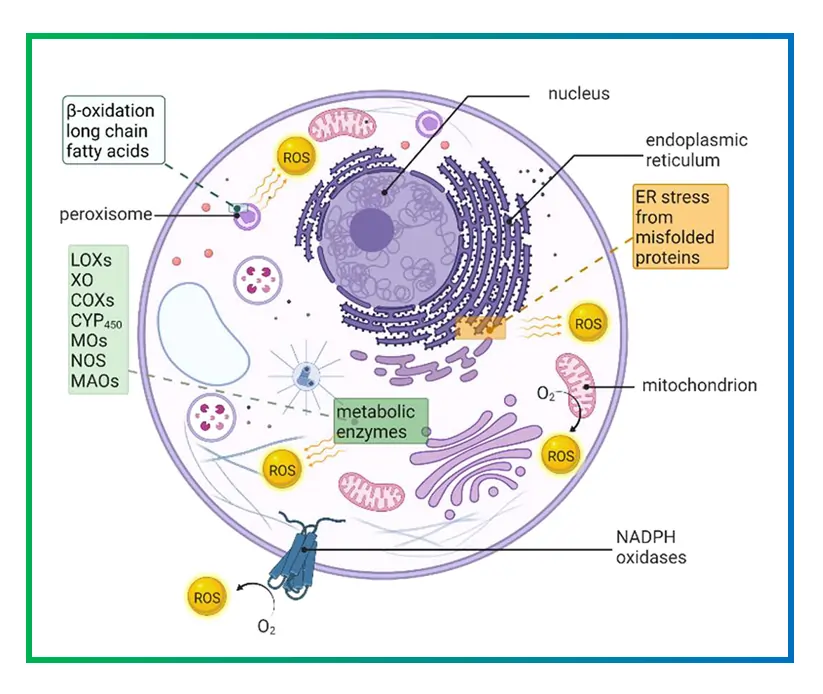
Reactive oxygen species include things like superoxide anions, hydroxyl radicals, and hydrogen peroxide. They’re created during normal cell processes, but their levels need to be tightly controlled. When ROS build up, they can damage proteins, DNA, and lipids, leading to conditions like inflammation or cell death.
Think of ROS as messengers in the body. In small amounts, they signal cells to adapt or fight infections. For example, in immune cells, ROS help kill bacteria during an infection. But if the balance tips, it results in oxidative stress, which is linked to aging, heart disease, and even cancer. Studies have shown that enzymes like NOXs are primary sources of these ROS in many tissues, making them hot targets for drug development.
One key reaction involving NOXs is the generation of superoxide:
$$ 2O_2 + NADPH \to 2O_2^{\bullet-} + NADP^+ + H^+ $$
This equation shows how NADPH donates electrons to oxygen, producing the superoxide radical. From there, superoxide can convert to other ROS like hydrogen peroxide through enzymatic reactions.
The Discovery and Family of NADPH Oxidases
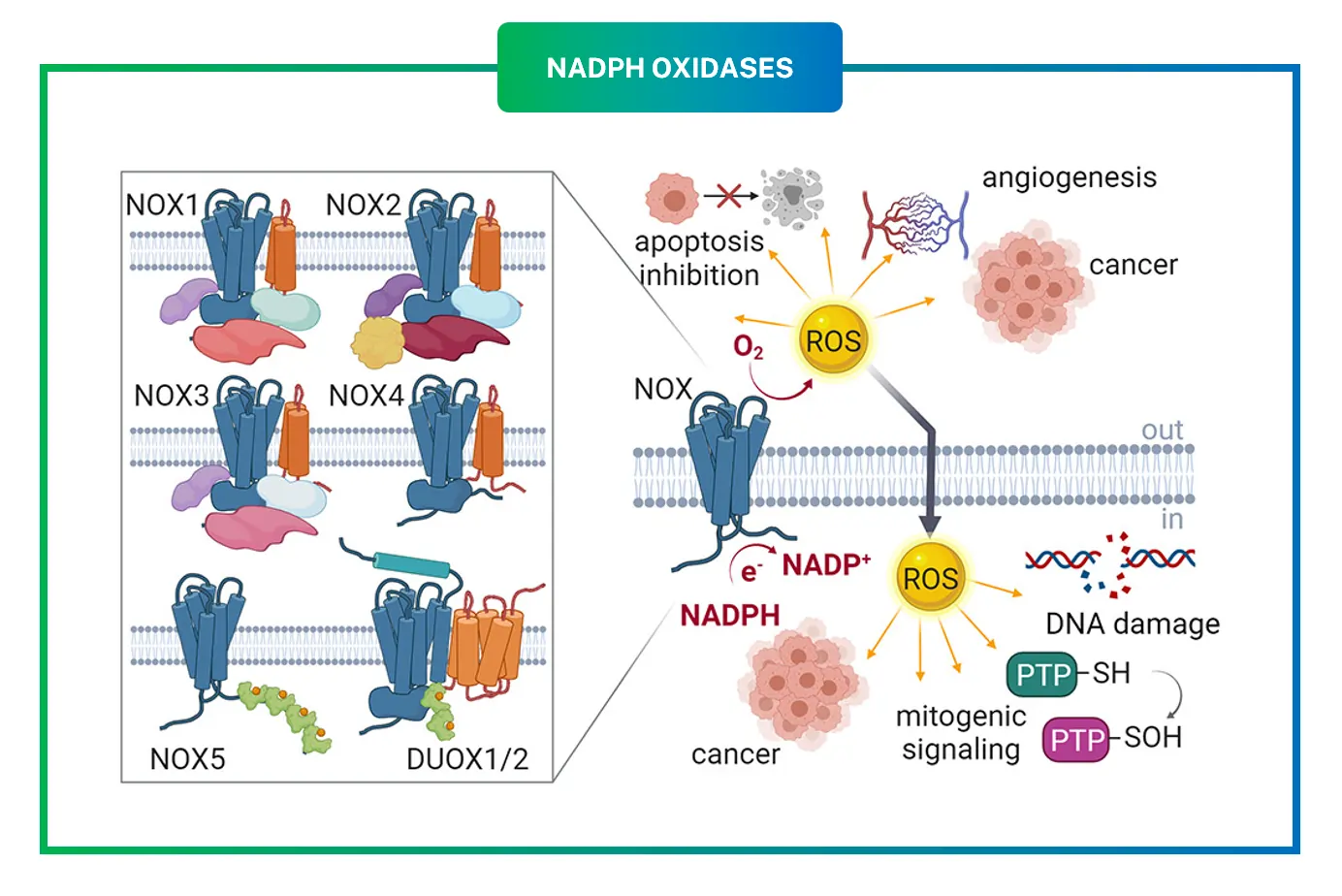
The story of NOXs started with observations in immune cells, where a “respiratory burst” produces massive ROS to combat invaders. The first identified was NOX2, originally called gp91phox, found in neutrophils. Over time, researchers uncovered six more: NOX1, NOX3, NOX4, NOX5, DUOX1, and DUOX2. Each has unique roles based on where they’re expressed in the body.
- NOX1: Mostly in colon epithelial cells, involved in host defense and cell proliferation.
- NOX2: Key in phagocytes like macrophages, central to immune responses.
- NOX3: Found in the inner ear, important for balance and hearing.
- NOX4: Widespread in kidneys and blood vessels, produces hydrogen peroxide steadily.
- NOX5: Calcium-regulated, present in lymphoid tissues and testes.
- DUOX1 and DUOX2: Dual oxidases with peroxidase domains, crucial in thyroid hormone synthesis and airway defense.
These enzymes aren’t just random; their diversity allows tailored ROS production in different organs. For instance, mutations in NOX2 cause chronic granulomatous disease, where patients can’t fight infections properly.
Diving into the Structure of NOX Enzymes
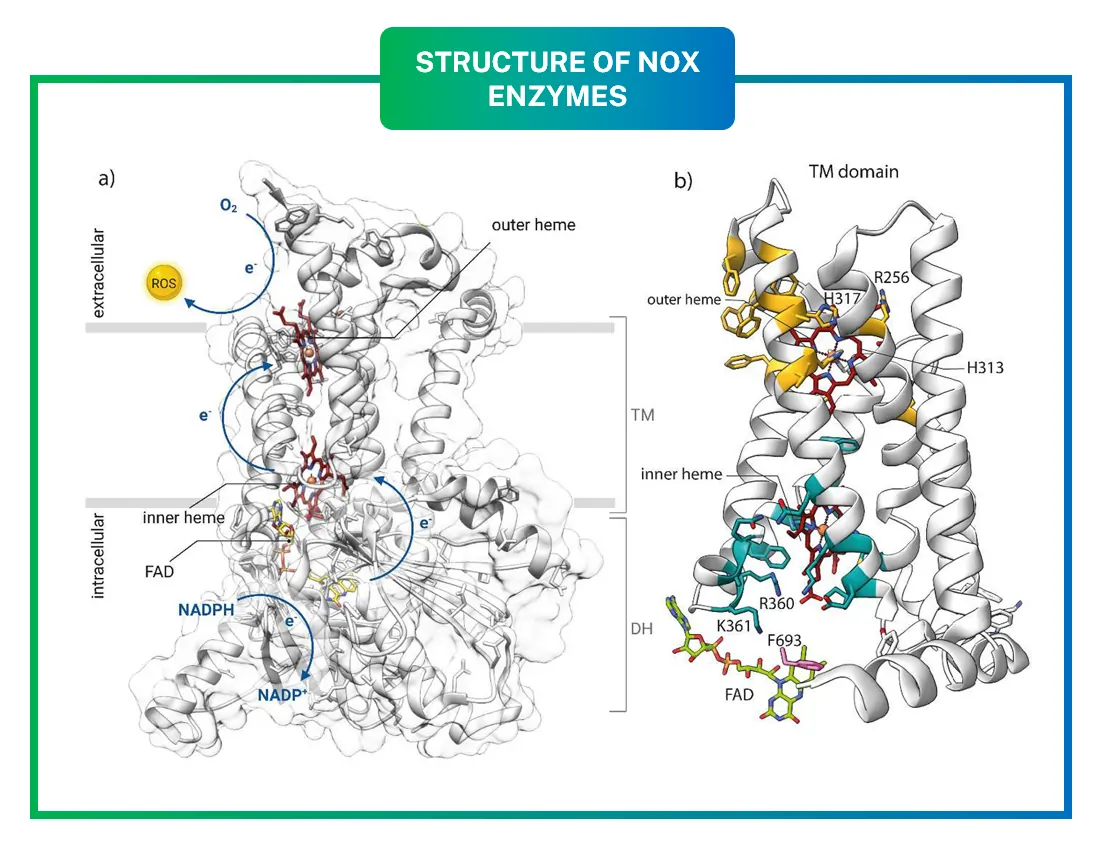
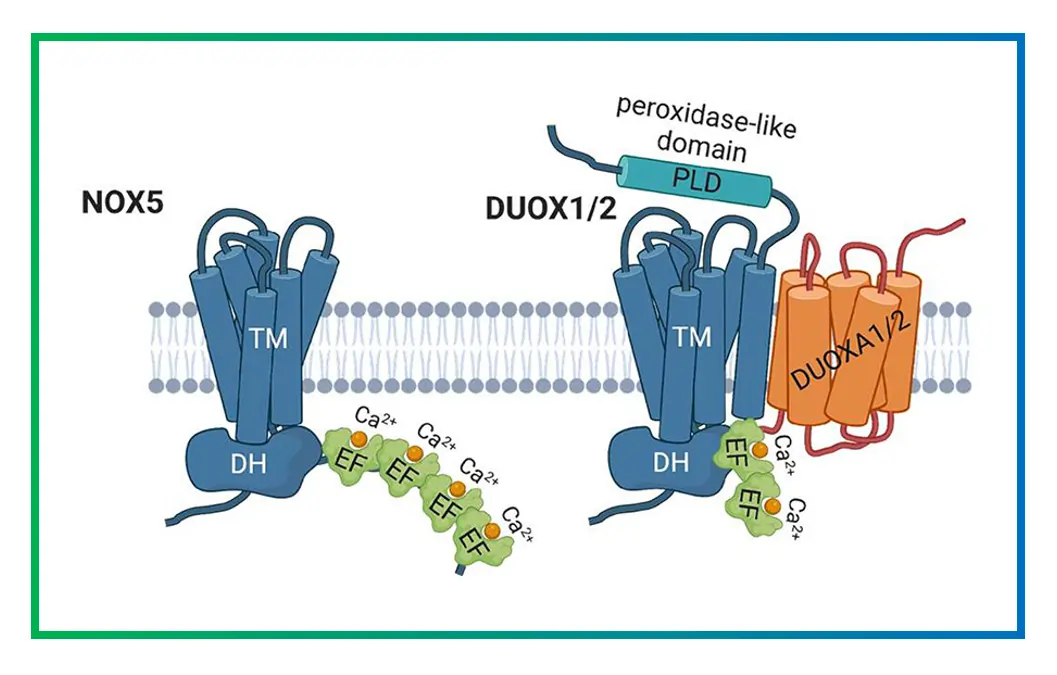
At their core, all NOXs share a similar architecture: a transmembrane domain (TM) and a cytosolic dehydrogenase domain (DH). The TM domain has six helices that span the cell membrane, holding two heme groups that ferry electrons. These hemes are coordinated by histidine residues, ensuring efficient electron transfer.
. 2023 Aug 31;66(17):11632–11655. doi: 10.1021/acs.jmedchem.3c00770)
The DH domain binds FAD and NADPH, kickstarting the process. Crystal structures, like those from NOX5 in algae, reveal how these parts fit together. The inner heme sits near the cytosol, while the outer one faces outside, allowing electrons to cross the membrane.
Imagine the enzyme as a tunnel: electrons enter from NADPH, pass through FAD, hop to the inner heme, then the outer heme, and finally reduce oxygen to ROS. A conserved arginine residue helps stabilize the oxygen-binding pocket, boosting efficiency.
Recent cryo-EM studies on DUOX1 and DUOX2 have added more details, showing how calcium-binding sites in NOX5 and DUOXs regulate activity. These structures highlight potential drug-binding sites, like the interface between DH and TM domains.
How NOX Enzymes Are Regulated
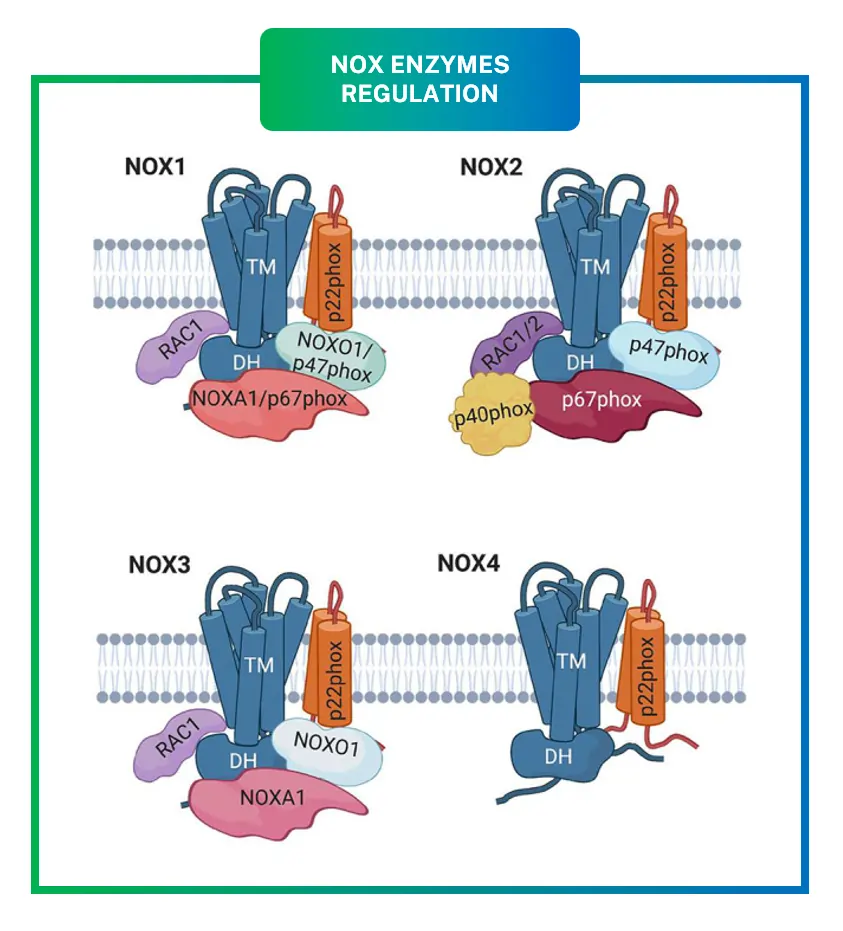
Regulation is key to preventing runaway ROS production. NOX1-4 require accessory subunits for activation, while NOX5 and DUOXs are more independent.
For NOX1, subunits like NOXO1 and NOXA1 organize the complex, similar to NOX2‘s p47phox and p67phox. Phosphorylation by kinases like PKC triggers assembly, moving subunits to the membrane.
. 2023 Aug 31;66(17):11632–11655. doi: 10.1021/acs.jmedchem.3c00770)
NOX4 is constitutively active, producing H2O2 directly, which is less damaging than superoxide. It’s regulated by expression levels rather than assembly.
DUOX1/2 have extra peroxidase domains that convert superoxide to H2O2, and they’re activated by calcium via EF-hand motifs.
Environmental factors like cytokines or growth factors can upregulate NOXs. In diabetes, high glucose activates NOX1 in vessels, leading to endothelial damage.
. 2023 Aug 31;66(17):11632–11655. doi: 10.1021/acs.jmedchem.3c00770)
NOXs in Health and Disease: Key Examples
NOXs are vital for normal functions but implicated in many diseases when dysregulated.
In cardiovascular health, NOX2 and NOX4 contribute to atherosclerosis by oxidizing LDL cholesterol, promoting plaque buildup. Studies link NOX1 to hypertension through vascular remodeling.
Cancer is another area: NOX1 and NOX4 generate ROS that promote tumor growth and metastasis. For example, in colon cancer, NOX1 overexpression correlates with poor prognosis.
Neurodegenerative disorders like Alzheimer’s involve NOX2-driven ROS in microglial activation, damaging neurons.
Inflammation ties back to NOX2 in immune cells, but chronic activation leads to autoimmune issues.
Fibrosis in lungs or liver often stems from NOX4-mediated ROS, activating fibroblasts.
Therapeutic targeting could help. Animal models show knocking out NOX2 reduces stroke damage, hinting at inhibitor potential.
Challenges in Developing NOX Inhibitors
Finding selective inhibitors is tough because NOXs share similar catalytic sites. Early compounds like DPI inhibit broadly, causing off-target effects.
Ideally, inhibitors should target specific isoforms without affecting others. High-throughput screening and structure-based design are advancing this.
Biological assays include measuring ROS with probes like DCFH-DA in cells, or enzymatic activity in membranes. In vivo, animal models of disease test efficacy.
Current Inhibitors: A Detailed Look
Over the last two decades, many small-molecule inhibitors have emerged. Let’s break them down by category.
Non-Selective Inhibitors
These hit multiple NOXs but provide starting points.
- Diphenyleneiodonium (DPI): A classic flavin analog, blocks electron transfer. Used in research but toxic in vivo.
- Apocynin: From plants, inhibits assembly by blocking subunit translocation. Effective in vascular models but metabolized oddly.
NOX2-Specific Inhibitors
Targeting immune-related diseases.
- GSK2795039: Orally bioavailable, reduces ROS in pancreatitis models. IC50 around 250 nM.
- Phox-I1: Peptidomimetic, disrupts p47phox-p22phox interaction.
NOX4 Inhibitors
Promising for fibrosis.
- GKT137831 (Setanaxib): Dual NOX1/4 inhibitor, in clinical trials for liver fibrosis. Reduces oxidative stress in diabetic kidneys.
- GKT136901: Similar, but less advanced.
NOX1 Inhibitors
For cancer and gut issues.
- ML171: Selective, blocks colon ROS production.
NOX5 and DUOX Inhibitors
Less developed, but NOX5 inhibitors like VAS2870 show calcium-dependent blockade.
Many inhibitors work by binding the DH domain or disrupting subunit interactions. Virtual screening using PDB structures accelerates discovery.
Table of NOX Isoforms and Their Characteristics
| Isoform | Tissue Expression | Main ROS Produced | Regulatory Subunits | Key Diseases Involved | Activation Mechanism |
|---|---|---|---|---|---|
| NOX1 | Colon, vascular smooth muscle | Superoxide | NOXO1, NOXA1, p22phox | Hypertension, colon cancer | Phosphorylation, Rac GTPase |
| NOX2 | Phagocytes, endothelium | Superoxide | p22phox, p47phox, p67phox, p40phox, Rac | Chronic granulomatous disease, atherosclerosis | Assembly upon stimulation |
| NOX3 | Inner ear, fetal tissues | Superoxide | NOXO1, p22phox | Hearing loss | Constitutive with subunits |
| NOX4 | Kidney, vessels, heart | Hydrogen peroxide | p22phox | Fibrosis, diabetic nephropathy | Constitutive, expression-regulated |
| NOX5 | Lymphocytes, testes, spleen | Superoxide | None (calcium-binding) | Cancer, cardiovascular | Calcium and phosphorylation |
| DUOX1 | Airways, salivary glands | Hydrogen peroxide | DUOXA1 | Infections, hypothyroidism | Calcium, peroxidase domain |
| DUOX2 | Thyroid, gastrointestinal | Hydrogen peroxide | DUOXA2 | Congenital hypothyroidism | Similar to DUOX1 |
This table summarizes the diversity, helping understand why selective targeting matters.
Table of Selected NOX Inhibitors
| Inhibitor Name | Target Isoforms | Mechanism of Action | IC50 (nM) | In Vitro Assays | In Vivo Studies | Potential Side Effects |
|---|---|---|---|---|---|---|
| DPI | All NOXs | Flavin binding | 10-100 | ROS fluorescence, EPR | Limited due to toxicity | Mitochondrial inhibition |
| Apocynin | NOX1-4 | Assembly disruption | 10000 | Neutrophil burst assay | Hypertension models | Non-specific oxidation |
| GSK2795039 | NOX2 | Unknown, selective | 250 | Cell-based ROS | Pancreatitis in mice | Minimal reported |
| GKT137831 | NOX1/4 | DH domain binding | 140/90 | Amplex Red for H2O2 | Fibrosis trials | Well-tolerated in humans |
| VAS2870 | Pan-NOX | Covalent binding | 200-1000 | Lucigenin chemiluminescence | Cancer xenografts | Off-target on kinases |
| ML171 | NOX1 | Selective blocker | 130 | HEK293 expression system | Gut inflammation | Low toxicity |
| Phox-I1 | NOX2 | Protein-protein interaction | 1000 | Pull-down assays | Inflammatory models | Peptide stability issues |
These tables highlight progress, with some like GKT137831 advancing to phase II trials.
Future Directions and Therapeutic Potential
Looking ahead, combining inhibitors with antioxidants could enhance efficacy. Gene therapies targeting specific NOXs are emerging, especially for rare diseases.
Challenges remain: ensuring selectivity to avoid disrupting beneficial ROS signaling. Biomarkers for NOX activity would help monitor treatments.
In summary, NOXs bridge normal physiology and pathology, offering exciting drug targets. Ongoing research promises better inhibitors, potentially transforming treatments for oxidative stress-related diseases.
This field evolves rapidly, with new structures and compounds yearly. Staying informed could lead to breakthroughs in personalized medicine.
Frequently Asked Questions
FAQ 1: What are the primary functions of NADPH oxidases in maintaining human health?
NADPH oxidases, commonly known as NOX enzymes, serve as vital components in the body’s defense and signaling systems by producing reactive oxygen species. These enzymes are embedded in cell membranes and actively generate molecules like superoxide and hydrogen peroxide, which act as signaling agents in various physiological processes.
For instance, in immune cells such as neutrophils and macrophages, NOX2 plays a starring role during the respiratory burst, where it floods invading pathogens with reactive oxygen species to neutralize them effectively. This mechanism is essential for innate immunity, helping the body fend off bacterial and fungal infections without relying solely on other immune responses.
Beyond immunity, NOX enzymes contribute to cell homeostasis by regulating redox states, which influence everything from gene expression to protein activation. In vascular tissues, for example, NOX1 and NOX4 help maintain blood vessel tone and respond to shear stress, ensuring proper blood flow and preventing issues like hypertension. Research highlights their involvement in developmental processes too, such as in the inner ear where NOX3 aids in the formation of otoconia, tiny crystals crucial for balance and hearing. Without balanced NOX activity, these functions could falter, leading to disorders ranging from chronic infections to sensory impairments.
Interestingly, the dual nature of reactive oxygen species means NOX enzymes also participate in adaptive responses to stress. In moderate amounts, the reactive oxygen species they produce can promote cell survival and proliferation, as seen in wound healing where they signal fibroblasts to repair tissue. However, this balance is delicate; overactivation can tip into pathology, but in health, NOX enzymes ensure a controlled environment for cellular communication and protection.
FAQ 2: How do NADPH oxidases produce reactive oxygen species at the molecular level?
The production of reactive oxygen species by NADPH oxidases begins with the enzyme’s core structure, which facilitates electron transfer across cell membranes. At the heart of this process is the transmembrane domain with its six helices and two heme groups, working in tandem with the cytosolic dehydrogenase domain that binds NADPH and FAD. When activated, NADPH donates electrons to FAD, reducing it to FADH2, and these electrons then hop sequentially to the inner heme, outer heme, and finally to molecular oxygen on the extracellular side.
This electron shuttle results in the reduction of oxygen to superoxide anion, which can further convert to other reactive species like hydrogen peroxide. The key reaction can be represented as:
$$ \text{NADPH} + 2O_2 \to \text{NADP}^+ + 2O_2^{\bullet-} + H^+ $$
Such a mechanism ensures targeted reactive oxygen species generation, often triggered by specific stimuli like calcium for NOX5 or subunit assembly for NOX2. Recent structural studies using cryo-EM have refined our understanding, showing how conserved residues like arginine in the oxygen-binding pocket stabilize the process and enhance superoxide output.
In practical terms, this controlled production is what allows reactive oxygen species to function as messengers rather than mere byproducts. For example, in endothelial cells, the steady hydrogen peroxide from NOX4 influences gene transcription for antioxidants, creating a feedback loop that maintains cellular balance.
FAQ 3: What are the various isoforms of NADPH oxidases and their specific characteristics?
NADPH oxidases come in seven isoforms, each with unique expressions, products, and roles, making them adaptable to different bodily needs. Understanding these differences is key for targeted therapies in diseases where specific isoforms dominate.
| Isoform | Primary Tissue Expression | Main Reactive Oxygen Species Produced | Key Regulatory Mechanisms | Associated Physiological Roles | Linked Pathologies |
|---|---|---|---|---|---|
| NOX1 | Colon epithelium, vascular smooth muscle | Superoxide | Phosphorylation via PKC, Rac GTPase involvement | Host defense in gut, vascular remodeling | Hypertension, colon cancer progression |
| NOX2 | Phagocytes like neutrophils, endothelium | Superoxide | Subunit assembly (p47phox, p67phox), phosphorylation | Immune response via respiratory burst, pathogen clearance | Chronic granulomatous disease, atherosclerosis |
| NOX3 | Inner ear, fetal kidney | Superoxide | Constitutive with NOXO1 and p22phox | Otoconia formation for balance and hearing | Vestibular dysfunction, hearing loss |
| NOX4 | Kidney, heart, vessels | Hydrogen peroxide primarily | Expression level regulation, constitutive activity | Steady reactive oxygen species for signaling, fibrosis control | Diabetic nephropathy, cardiac fibrosis |
| NOX5 | Lymph nodes, testes, spleen | Superoxide | Calcium binding via EF-hands, phosphorylation | Sperm motility, lymphoid signaling | Certain cancers, cardiovascular issues |
| DUOX1 | Airways, salivary glands | Hydrogen peroxide | Calcium activation, maturation factors | Mucosal defense, peroxide for antimicrobial action | Respiratory infections, hypothyroidism if dysregulated |
| DUOX2 | Thyroid, gastrointestinal tract | Hydrogen peroxide | Similar to DUOX1, with peroxidase domain | Thyroid hormone synthesis, gut barrier function | Congenital hypothyroidism, thyroid dyshormonogenesis |
This table underscores the diversity, with recent research emphasizing NOX4’s role in chronic conditions and DUOX isoforms in hormone production.
FAQ 4: How are NADPH oxidases regulated to prevent excessive reactive oxygen species production?
Regulation of NADPH oxidases is a sophisticated process that ensures reactive oxygen species are produced only when needed, avoiding oxidative damage. For isoforms like NOX1 through NOX4, regulation often involves accessory subunits that assemble upon specific signals. Take NOX2, for instance: it’s dormant until phosphorylation events, triggered by cytokines or pathogens, prompt subunits such as p47phox and p67phox to translocate to the membrane, forming an active complex with the catalytic core.
- Calcium plays a pivotal role in NOX5 and DUOX enzymes, binding to EF-hand motifs to induce conformational changes that activate electron transfer.
- Post-translational modifications, including phosphorylation by kinases like PKC or ubiquitination, fine-tune activity and degradation.
- Transcriptional control affects isoforms like NOX4, where factors such as TGF-beta upregulate expression in response to injury or inflammation.
- Negative feedback loops, involving antioxidants or heme oxygenase-1, can dampen activity to restore balance.
These mechanisms highlight the enzyme’s adaptability, with recent studies showing how evolutionary adaptations have added layers like peroxidase domains in DUOX for direct hydrogen peroxide handling.
FAQ 5: What diseases are linked to the dysregulation of NADPH oxidases?
Dysregulation of NADPH oxidases has been implicated in a wide array of diseases, primarily through excessive reactive oxygen species leading to oxidative stress. In cardiovascular conditions, overactive NOX1 and NOX2 contribute to atherosclerosis by oxidizing lipids and promoting inflammation in vessel walls, which can lead to plaque formation and heart attacks. Similarly, in hypertension, these enzymes exacerbate vascular remodeling, making blood vessels stiffer and less responsive.
Moving to metabolic disorders, diabetic complications often stem from NOX4’s heightened activity in kidneys and eyes, where it drives fibrosis and tissue damage. Research from recent years points to NOX enzymes in neurodegenerative diseases too, with NOX2 in microglia amplifying neuroinflammation in Alzheimer’s and Parkinson’s, accelerating neuron loss.
Cancer represents another major area, as NOX-generated reactive oxygen species can promote tumor growth by mutating DNA or activating survival pathways. For example, in colon cancer, NOX1 overexpression correlates with metastasis. Infectious and inflammatory diseases also feature prominently; while NOX2 is crucial for fighting infections, its deficiency causes chronic granulomatous disease, leaving individuals vulnerable to recurrent infections.
Emerging evidence ties NOX to conditions like fibrosis in lungs and liver, where persistent activation leads to scar tissue buildup. In the context of recent global health challenges, studies suggest NOX involvement in severe inflammatory responses during viral infections, underscoring the need for balanced activity.
FAQ 6: What are some promising inhibitors of NADPH oxidases currently under investigation?
Inhibitors of NADPH oxidases hold great potential for treating oxidative stress-related diseases, with several advancing through research and trials. Here’s a detailed overview of key ones, including their targets and status.
| Inhibitor | Targeted Isoforms | Mechanism | Key Findings from Studies | Clinical Trial Status | Potential Applications |
|---|---|---|---|---|---|
| Setanaxib (GKT137831) | NOX1 and NOX4 | Binds to dehydrogenase domain, blocks electron transfer | Reduces fibrosis in animal models of liver and kidney disease | Phase II/III for primary biliary cholangitis and diabetic kidney disease | Fibrotic disorders, diabetic complications |
| GSK2795039 | Primarily NOX2 | Selective inhibition, reduces superoxide in immune cells | Effective in pancreatitis and inflammatory models | Preclinical to early clinical | Inflammatory diseases, acute pancreatitis |
| APX-115 | Pan-NOX, focuses on NOX1/4 | Antioxidant modulation | Protects against oxidative damage in metabolic syndromes | Pipeline for replacement therapies | Diabetes, cardiovascular issues |
| VAS2870 | Broad NOX inhibition | Covalent binding to catalytic site | Suppresses reactive oxygen species in cancer xenografts | Preclinical | Cancer adjunct therapy |
| EN-374 | NOX replacement approach | Dual covalent on NOX2/MAOB | Potent in reducing oxidative stress in neurological models | Early development | Neurodegenerative diseases |
| ML171 | NOX1 selective | Blocks assembly or activity | Mitigates gut inflammation | Preclinical | Inflammatory bowel disease |
These inhibitors reflect ongoing efforts, with some like Setanaxib showing tolerability in humans and progressing toward broader use.
FAQ 7: What recent advances have been made in understanding the structure of NADPH oxidases?
Recent structural biology has revolutionized our view of NADPH oxidases, providing atomic-level insights that were once elusive. Cryo-electron microscopy structures of human NOX2 and DUOX1/2 have revealed how the transmembrane and dehydrogenase domains interact, with the heme groups precisely positioned for electron flow. These updates show orthogonal heme orientation relative to the membrane, optimizing transfer efficiency.
Building on earlier X-ray crystallography of NOX5 fragments, newer models highlight regulatory elements like the D-loop’s conserved arginine, which stabilizes the interdomain interface. Such details explain why mutations disrupt function, as in chronic granulomatous disease.
Prokaryotic NOX studies offer evolutionary clues, with structures indicating simpler regulation that evolved into complex mammalian systems. These advances pave the way for structure-based drug design, targeting pockets like the oxygen-binding site for isoform-specific inhibitors.
FAQ 8: What is the evolutionary origin of NADPH oxidases across species?
NADPH oxidases trace their roots deep into evolutionary history, appearing in prokaryotes as simple electron transporters before diversifying in eukaryotes. In bacteria, ancestral NOX enzymes likely served basic redox functions, such as detoxifying oxygen or signaling in biofilms. As eukaryotes emerged, gene duplications led to specialized isoforms, with the core transmembrane and dehydrogenase domains conserved across kingdoms.
In mammals, the family expanded to seven members, incorporating regulatory features like calcium-binding EF-hands in NOX5, which probably arose from an early duplication event. DUOX enzymes represent a further evolution, adding peroxidase domains to directly manage hydrogen peroxide, essential for tissues like the thyroid.
This progression reflects adaptation to multicellular life, where controlled reactive oxygen species became crucial for immunity and development. For instance, in arthropods, unique isoforms like NOX4-art highlight neofunctionalization, emphasizing how evolution tailored these enzymes for diverse roles from microbial defense to hormone synthesis.
FAQ 9: Can natural compounds effectively inhibit NADPH oxidases?
Natural compounds have shown promising potential as inhibitors of NADPH oxidases, offering gentler alternatives to synthetic drugs for managing oxidative stress. Polyphenols from plants, such as resveratrol found in grapes and berries, interfere with subunit assembly in NOX1 and NOX2, reducing reactive oxygen species in vascular cells. Curcumin from turmeric similarly modulates NOX4 expression, helping in conditions like diabetes by curbing inflammation.
- Celastrol, derived from thunder god vine, potently blocks NOX activity at low concentrations, making it a candidate for anti-inflammatory therapies.
- Apocynin, originally from Picrorhiza kurroa, prevents p47phox translocation, though its metabolism requires careful dosing.
- Berberine from barberry plants downregulates NOX genes, aiding in metabolic disorders.
- Thymoquinone in black cumin seeds targets multiple isoforms, with antioxidant effects amplifying inhibition.
These compounds often work through multiple pathways, enhancing their appeal, but more clinical data is needed to confirm efficacy and safety.
FAQ 10: What is the significance of DUOX enzymes in thyroid hormone production?
DUOX enzymes, specifically DUOX1 and DUOX2, are indispensable for thyroid function, acting as the primary generators of hydrogen peroxide needed for hormone synthesis. In the thyroid gland, DUOX2 dominates, producing peroxide that thyroperoxidase uses to iodinate thyroglobulin, forming precursors to thyroid hormones like T3 and T4. This process ensures proper metabolism, growth, and development throughout the body.
Deficiencies in DUOX2, often genetic, lead to congenital hypothyroidism, where insufficient hormone production causes developmental delays if untreated. Recent insights show DUOX maturation factors are crucial for enzyme stability and activity, with calcium signaling triggering peroxide release.
Beyond the thyroid, DUOX1 supports mucosal defenses in airways, but in thyroid contexts, both isoforms provide redundancy. Dysregulation can contribute to thyroid dyshormonogenesis or even autoimmunity, highlighting their role in endocrine health.
FAQ 11: What is the role of NADPH oxidases in the immune system?
NADPH oxidases play a pivotal role in the body’s immune defense, acting as key enzymes that generate reactive oxygen species to combat infections. In phagocytic cells like neutrophils and macrophages, the primary isoform, NOX2, triggers a respiratory burst upon encountering pathogens. This burst involves the rapid production of superoxide anions, which then convert into other potent reactive oxygen species such as hydrogen peroxide and hypochlorous acid. These molecules damage bacterial cell walls, proteins, and DNA, effectively neutralizing threats and preventing the spread of infection. Without this mechanism, as seen in conditions like chronic granulomatous disease, individuals become highly susceptible to recurrent bacterial and fungal infections due to impaired pathogen clearance.
Beyond direct antimicrobial action, NADPH oxidases contribute to immune regulation by modulating inflammation and signaling pathways. For instance, reactive oxygen species from these enzymes influence the activation of transcription factors like NF-kappaB, which orchestrates the production of cytokines and chemokines essential for recruiting additional immune cells to infection sites. In adaptive immunity, they help in antigen processing and presentation, ensuring T cells and B cells respond appropriately. Recent studies emphasize their dual role: while essential for host defense, excessive activation can lead to tissue damage in chronic inflammatory states, highlighting the need for balanced activity.
In broader contexts, NADPH oxidases in non-phagocytic immune cells, such as dendritic cells, fine-tune responses to ensure tolerance and prevent autoimmunity. Emerging research shows they restrain excessive inflammation in organs like the lungs by modulating redox-sensitive pathways, preventing overzealous immune reactions that could harm healthy tissue.
FAQ 12: How do NOX enzymes differ from DUOX enzymes?
NOX and DUOX enzymes both belong to the NADPH oxidase family, but they exhibit distinct structural and functional characteristics that adapt them to specific biological roles. Understanding these differences is crucial for appreciating their contributions to reactive oxygen species production across various tissues.
| Feature | NOX Enzymes (NOX1-5) | DUOX Enzymes (DUOX1-2) |
|---|---|---|
| Structure | Comprise a core with transmembrane and dehydrogenase domains, plus regulatory subunits for most isoforms. Lack a peroxidase domain. | Include the standard NOX core but add an N-terminal peroxidase-like domain and EF-hand motifs for calcium binding. |
| Primary ROS Produced | Mainly superoxide anion, which can dismutate to hydrogen peroxide. | Directly produce hydrogen peroxide due to the peroxidase domain, reducing superoxide intermediates. |
| Activation Mechanism | Often require subunit assembly (e.g., p47phox for NOX2) or phosphorylation; NOX5 is calcium-sensitive without subunits. | Highly calcium-dependent via EF-hands; maturation factors like DUOXA1/2 are essential for proper folding and activity. |
| Tissue Expression | Widespread: NOX2 in phagocytes, NOX4 in kidneys, NOX1 in colon. | Primarily in epithelial tissues: DUOX1 in airways, DUOX2 in thyroid and gut. |
| Physiological Roles | Key in immune defense, vascular signaling, and cell proliferation. | Involved in mucosal defense, thyroid hormone synthesis, and extracellular matrix reactions. |
| Evolutionary Aspects | Ancestral forms in prokaryotes; diversified for targeted superoxide production. | Evolved with peroxidase for controlled peroxide generation, seen in higher eukaryotes. |
| Pathological Implications | Linked to inflammation, cancer, and cardiovascular diseases when overactive. | Associated with hypothyroidism and respiratory infections if mutated. |
These distinctions highlight how DUOX enzymes provide a more contained reactive oxygen species output, minimizing cellular damage compared to the burst-like activity of some NOX isoforms.
FAQ 13: What are common assays used to measure NADPH oxidase activity?
Measuring NADPH oxidase activity is essential for understanding its role in health and disease, with various assays providing insights into reactive oxygen species production. These methods range from simple spectrophotometric techniques to more sophisticated kits, each suited to different experimental needs.
- Cytochrome c Reduction Assay: This classic method detects superoxide by monitoring the reduction of cytochrome c at 550 nm. It’s widely used for cell-free systems but requires controls like superoxide dismutase to confirm specificity.
- Amplex Red Assay: Ideal for hydrogen peroxide detection, it involves horseradish peroxidase converting Amplex Red to fluorescent resorufin. Best for NOX4 activity, though limited in cell-free setups due to interference.
- Lucigenin Chemiluminescence: Measures superoxide via light emission from lucigenin oxidation. Sensitive for low-level activity but prone to artifacts, so often paired with inhibitors like DPI.
- XTT Formazan Assay: For plant or membrane preparations, it quantifies superoxide by XTT reduction to formazan, read at 470 nm. Fresh NADPH and XTT solutions are crucial for accuracy.
- NADP/NADPH Ratio Kits: Colorimetric or fluorometric kits like ab65349 assess total NADP/NADPH levels, indirectly reflecting oxidase consumption. Useful for intracellular nucleotide detection.
- Electron Paramagnetic Resonance (EPR): Advanced spin-trapping technique for direct ROS identification, often in research settings to distinguish superoxide from other radicals.
- Isotopic Assays: Track NADP formation via 6-phosphogluconate dehydrogenase, providing precise quantification in complex samples.
These assays help researchers correlate enzyme activity with pathological states, with recent developments focusing on real-time monitoring in live cells.
FAQ 14: Are there any potential side effects of using NOX inhibitors?
Using NOX inhibitors holds promise for treating oxidative stress-related conditions, but they come with potential side effects that warrant careful consideration. Since NOX enzymes are integral to normal physiological processes, broad inhibition can disrupt beneficial reactive oxygen species signaling. For example, compounds like DPI, while effective in lab settings, have shown toxicity in vivo, including hypoglycemia and cardiomyopathy after prolonged use, stemming from off-target effects on mitochondria and other flavin-dependent enzymes.
In animal models, systemic deletion or inhibition of specific isoforms like NOX4 has led to increased susceptibility to injuries in organs such as the kidneys, heart, and vessels. This suggests that blocking NOX could exacerbate conditions like acute tubular injury or atherosclerosis by impairing adaptive responses. Clinical trials of inhibitors like Setanaxib have reported good tolerability, but concerns remain about long-term impacts on immune function, as reduced reactive oxygen species might weaken pathogen clearance, potentially increasing infection risks.
Moreover, some inhibitors exhibit assay-interfering properties or ROS scavenging effects that complicate their specificity, leading to unintended modulation of other pathways. In neurological contexts, over-inhibition might affect pain processing or exacerbate brain injury, while in metabolic disorders, it could influence insulin signaling adversely. Overall, the challenge lies in achieving isoform-selective inhibition to minimize these risks, with ongoing research aiming to refine dosing and delivery for safer therapeutic applications.
FAQ 15: How are NADPH oxidases involved in the aging process?
NADPH oxidases contribute significantly to aging by influencing oxidative stress levels, which accumulate over time and affect cellular function. As organisms age, upregulated NOX activity, particularly in isoforms like NOX4, leads to heightened reactive oxygen species in tissues such as the heart and kidneys, promoting cellular senescence and tissue decline. This process links to age-related cardiovascular changes, where ROS from NOX enzymes oxidize lipids and proteins, accelerating vascular stiffness and dysfunction.
- Mitochondrial interplay: NOX-derived ROS can amplify mitochondrial dysfunction, a hallmark of aging, creating a vicious cycle of oxidative damage.
- Renal aging: In kidneys, NOX isoforms exacerbate fibrosis and glomerular injury under normal and diabetic conditions, hastening organ deterioration.
- Cardiac implications: Upregulated NOX4 in aging hearts contributes to hypertrophy and failure, with inhibitors like apocynin showing potential to reverse some effects.
- Theoretical perspectives: Some theories posit NOX as central to free radical aging hypotheses, with ROS acting as signals that disrupt homeostasis.
- Therapeutic angles: Targeting NOX could mitigate age-related diseases, as transient ROS bursts help re-establish balance during aging.
These insights underscore NOX enzymes as modulators of longevity, with balanced activity key to healthy aging.
FAQ 16: What genetic mutations affect NADPH oxidase function?
Genetic mutations in NADPH oxidase genes can severely impair enzyme function, leading to disorders like chronic granulomatous disease. Below is a summary of key mutations and their impacts.
| Gene | Isoform Affected | Common Mutations | Functional Impact | Associated Conditions |
|---|---|---|---|---|
| CYBB | NOX2 (gp91phox) | Missense, nonsense, deletions (e.g., X910, X91−) | Absent or reduced superoxide production, impaired assembly | X-linked chronic granulomatous disease, recurrent infections |
| NCF1 | p47phox | GT deletion at exon 2, missense variants | Defective subunit translocation, low residual activity | Autosomal recessive chronic granulomatous disease |
| NCF2 | p67phox | Novel mutations like in CGD with colitis | Disrupted activation complex, variable ROI production | Chronic granulomatous disease with inflammatory bowel disease |
| NCF4 | p40phox | Rare variants | Altered phagosome regulation | Milder chronic granulomatous disease forms |
| CYBA | p22phox | Point mutations | Unstable core complex | Autosomal recessive chronic granulomatous disease |
| NOX3 | NOX3 | Mutations impairing SHH signaling | Increased cell proliferation, developmental defects | Ataxia, hearing and balance issues |
| DUOX2 | DUOX2 | Biallelic mutations | Deficient peroxide for hormone synthesis | Congenital hypothyroidism |
These mutations often predict disease severity based on residual activity, influencing survival and treatment strategies.
FAQ 17: How do NADPH oxidases compare to other sources of reactive oxygen species?
NADPH oxidases stand out as regulated sources of reactive oxygen species, differing from more constitutive producers like mitochondria. While mitochondria generate ROS as byproducts of electron transport, often leading to unintended oxidative damage, NADPH oxidases produce them deliberately for signaling and defense. For instance, in vascular cells, NOX enzymes are major superoxide contributors, especially in hypertensive states, surpassing mitochondrial output in certain contexts.
In macrophages, both sources coexist, with NADPH oxidases providing rapid bursts via endosomal localization, while mitochondria sustain longer-term ROS. This interplay can amplify effects, as NOX-derived ROS influence mitochondrial function, creating feedback loops in inflammation. Other enzymes like xanthine oxidase or uncoupled nitric oxide synthase also produce ROS, but NADPH oxidases are unique in their membrane-spanning electron transfer, enabling targeted extracellular release.
Comparatively, in skin exposed to UVA, NOX1 dominates ROS production over other sources, highlighting isoform-specific roles. Overall, NADPH oxidases offer controlled, stimulus-responsive generation, making them prime targets for modulation unlike the broader, less tunable mitochondrial ROS.
FAQ 18: What is the role of NADPH oxidases in plant biology?
In plant biology, NADPH oxidases, known as respiratory burst oxidase homologs or RBOHs, are central to reactive oxygen species production, serving as vital performers in stress responses and development. These enzymes generate superoxide, which dismutes to hydrogen peroxide, acting as signals for processes like pathogen defense and stomatal closure. The reaction can be expressed as:
$$ \text{NADPH} + 2O_2 \to \text{NADP}^+ + 2O_2^{\bullet-} + H^+ $$
This controlled output helps plants adapt to environmental challenges, such as drought or infection, by triggering gene expression for protective compounds.
Structurally similar to animal NOX, plant RBOHs feature calcium-binding EF-hands, allowing rapid activation by cytosolic calcium spikes. In Arabidopsis, isoforms like RBOHD mediate systemic signaling during wounding or herbivory, propagating waves of ROS across the plant. They also regulate cell growth, with mutations affecting pollen tube elongation or root hair development, underscoring their role in morphogenesis.
Beyond defense, RBOHs influence carbon metabolism and the cell cycle in algae like Chlamydomonas, where they link ROS to energy pathways. Post-translational modifications, including phosphorylation, fine-tune their activity, making them hubs for integrating signals from hormones like abscisic acid. This versatility positions NADPH oxidases as key players in plant resilience and evolution.
FAQ 19: What are future research directions for NADPH oxidases?
Future research on NADPH oxidases promises to unlock new therapeutic avenues, focusing on isoform-specific roles and regulatory mechanisms. As understanding deepens, directions include:
- Developing selective inhibitors: Prioritizing compounds that target individual isoforms without off-target effects, using structure-based design from recent cryo-EM data.
- Exploring neuronal development: Investigating NOX involvement in brain maturation and disorders, with emphasis on isoforms like NOX1-4 and DUOX1 in neurogenesis.
- Redox signaling in algae: Delving into unique pathways in red algae, where NOX regulates stress responses, to inform broader evolutionary insights.
- Post-translational modifications: Studying acetylation and deacetylation’s impact on activity, potentially revealing new control points for superoxide production.
- Clinical applications in cardiovascular disease: Clarifying Ang II and ROS interactions in vessels, guiding treatments for hypertension and atherosclerosis.
- NOX5 specifics: Addressing its out-of-context activation in diseases, aiming for context-specific modulators.
- p47phox therapies: Advancing regulators of this subunit for inflammation control, tackling challenges in delivery and efficacy.
These paths aim to harness NOX for interventions in aging, cancer, and neurological conditions.
FAQ 20: How have NADPH oxidases been linked to recent viral diseases like COVID-19?
NADPH oxidases have emerged as key players in the pathology of COVID-19, where SARS-CoV-2 infection triggers excessive reactive oxygen species production, exacerbating inflammation. In infected cells, viral proteins like the spike stimulate NOX2 in macrophages and microglia, leading to a ROS storm that promotes cytokine release and tissue damage, particularly in the lungs and brain. This activation via pathways like TLR4 and TLR7/8 amplifies neutrophil extracellular traps formation, contributing to thrombosis and severe respiratory distress.
In diabetic patients, pre-activated vascular NOX heightens vulnerability, linking to worse outcomes through endothelial dysfunction and reduced nitric oxide. Studies show that infection-induced ROS from NOX disrupts antioxidant balance, fostering a pro-inflammatory environment that worsens multi-organ failure. Interestingly, NADPH’s role in reducing severity suggests a dual aspect, where balanced oxidase activity might aid recovery.
Therapeutic implications include targeting NOX to mitigate neuroinflammation and oxidative stress in long COVID, with inhibitors potentially curbing the cytokine storm without compromising antiviral defenses.
Acknowledgments
The Examsmeta.com website extends its sincere gratitude to the global community of researchers and institutions whose groundbreaking work has shed light on the intricate world of NADPH oxidases and their pivotal roles in reactive oxygen species signaling and disease mechanisms. This article draws invaluable insights from peer-reviewed publications and expert resources, ensuring a comprehensive and evidence-based exploration of these essential enzymes.
Special thanks go to the National Institutes of Health (NIH) for foundational studies on oxidative stress pathways and enzyme regulation, the PubMed Central for open-access reviews on isoform-specific functions and therapeutic inhibitors, and the American Chemical Society (ACS) for detailed structural analyses that shaped our understanding of electron transfer mechanisms.
Additionally, Examsmeta appreciates the contributions from Nature Reviews Molecular Cell Biology on evolutionary origins and disease linkages, ScienceDirect for clinical trial data on NOX inhibitors, and Wikipedia for accessible overviews that guided initial framing—though all claims were rigorously cross-verified with primary sources. These collaborative efforts underscore the interdisciplinary nature of this field, and we remain committed to advancing knowledge for healthier futures.

