The world of cellular biology is full of fascinating enzymes that keep our bodies running smoothly, but few are as intriguing as the NADPH oxidase family. These enzymes are key players in producing reactive oxygen species, or ROS, which are molecules that can act as signals in cells or cause damage if they build up too much. Among them, NOX4 stands out because of its special traits and wide ranging effects on health.
In this article, we will dive deep into what makes NOX4 unique, how it works in the body, and its roles in various diseases. We will explore everything from its basic structure to potential treatments, drawing on the latest insights to give a clear picture. Whether you are curious about how cells handle oxygen or interested in new ways to fight diseases like cancer or heart problems, understanding NOX4 offers some exciting clues.
Table of Contents
Think about it: our cells need a delicate balance of ROS to function properly. Too little, and signaling pathways might not work right; too much, and it leads to oxidative stress, which is linked to aging, inflammation, and many chronic illnesses. NOX4 is like a steady worker in this system, constantly producing ROS without needing extra triggers, unlike some of its family members. This constant activity makes it vital in tissues like the heart, kidneys, and brain, where it helps with everything from blood vessel growth to sensing energy levels. But when things go wrong, NOX4 can tip the scales toward disease. Let us start by looking at the bigger picture of NADPH oxidases before zooming in on NOX4.
Introduction to NADPH Oxidases
NADPH oxidases are a group of seven enzymes in humans, named NOX1 through NOX5, plus DUOX1 and DUOX2. They all share the job of transferring electrons from NADPH to oxygen, creating ROS as a result. This process is crucial for fighting infections, as seen in immune cells where NOX2 produces superoxide to kill bacteria. But beyond defense, these enzymes help with cell signaling, growth, and even death when needed.
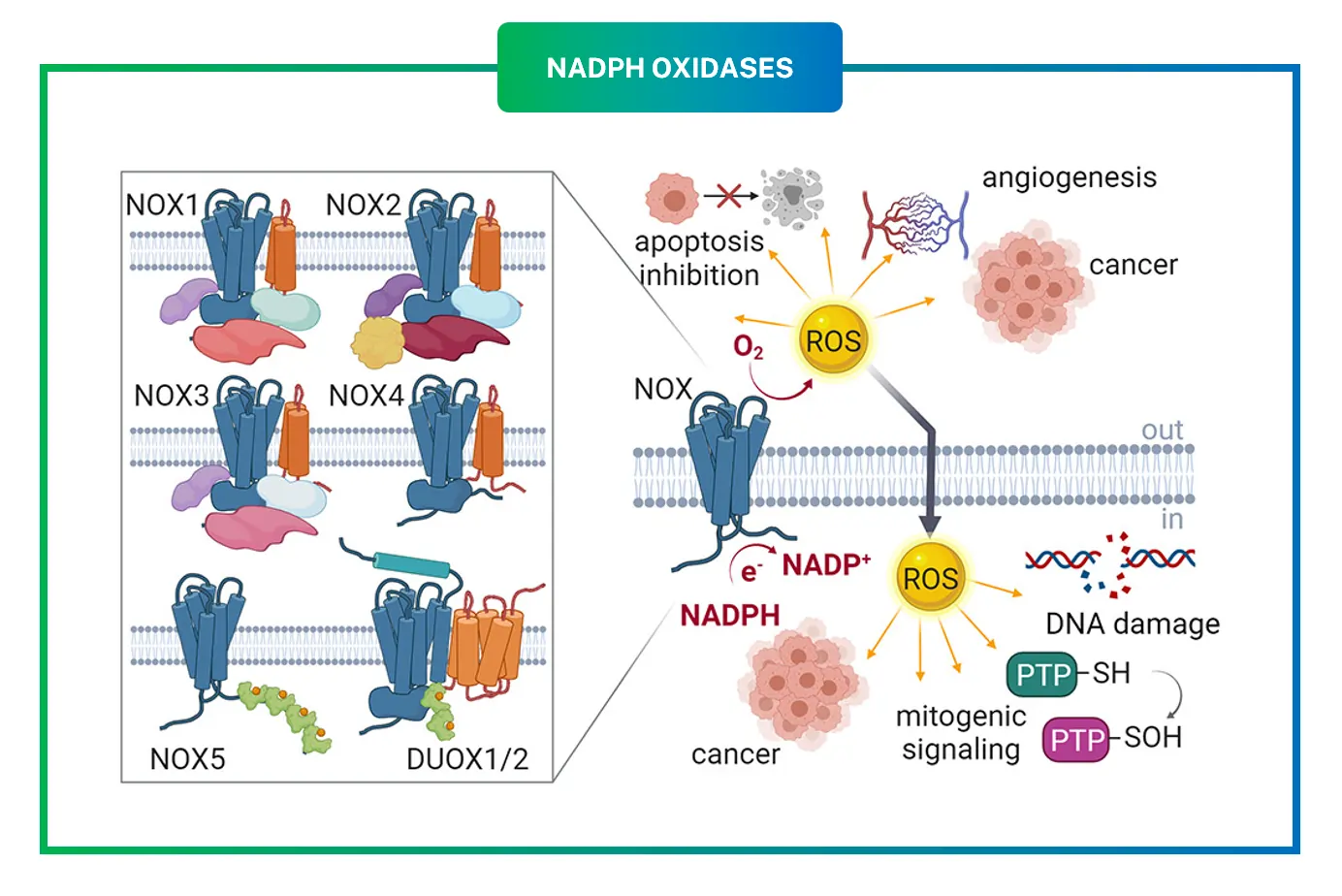
What sets the family apart is how each isoform behaves. Some need specific activators, while others are always on. They live in different parts of cells, like membranes or organelles, influencing where ROS are made and what they affect. For instance, in blood vessels, NADPH oxidases help control tone and repair. Over the years, researchers have linked their dysfunction to heart disease, cancer, and neurological issues. NOX4, in particular, has emerged as a star because it mostly makes hydrogen peroxide, a milder ROS that acts more like a messenger than a destroyer.
To give you a better sense, consider how these enzymes evolved. They likely started as tools for ancient cells to handle oxygen, turning a potential toxin into a useful signal. Today, imbalances in NADPH oxidase activity are tied to modern health problems, fueled by diets, stress, and aging. Studying them opens doors to new therapies, like drugs that tweak specific isoforms without affecting others.
What is NOX4? Structure and Mechanism
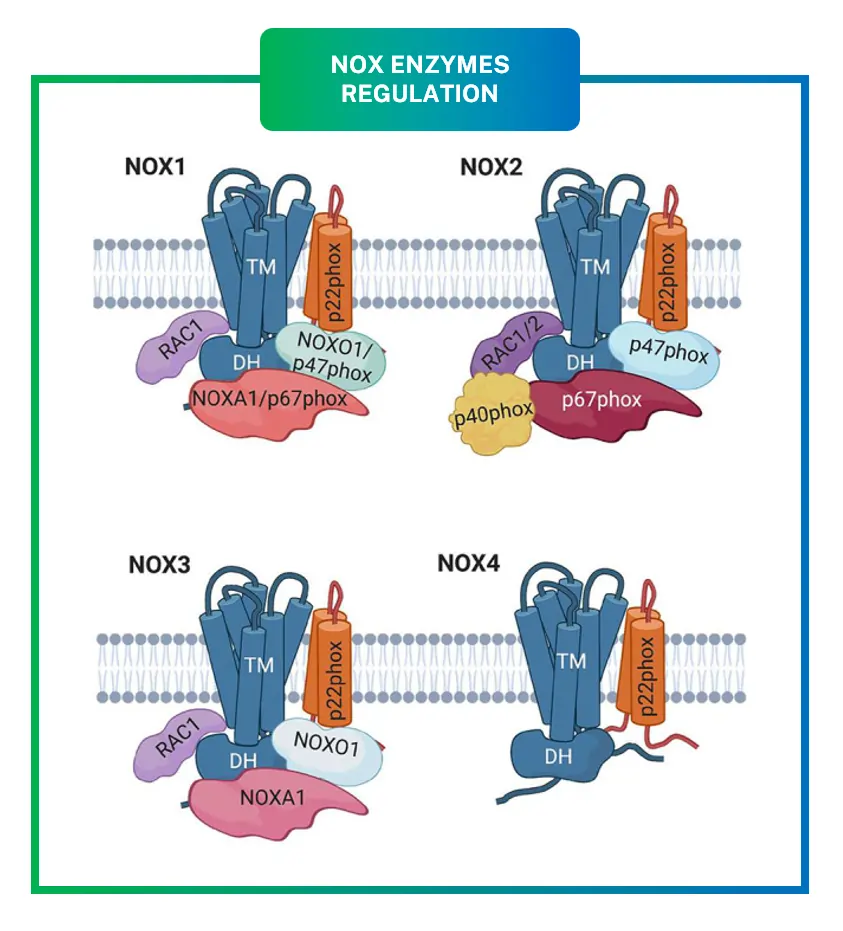
NOX4 is a transmembrane protein that spans cell membranes multiple times, with parts sticking out on both sides. It has six helical segments crossing the membrane, plus domains for binding NADPH and FAD, which help shuttle electrons. A key partner is p22phox, a smaller protein that stabilizes NOX4 and helps it fold properly. Unlike other isoforms, NOX4 does not need a bunch of extra proteins to turn on; it forms a simple duo with p22phox.
. 2023 Aug 31;66(17):11632–11655. doi: 10.1021/acs.jmedchem.3c00770)
The magic happens in its catalytic core. NOX4 takes electrons from NADPH on the inside of the cell and passes them to oxygen on the outside or in organelles. But here is the twist: while most NADPH oxidases spit out superoxide $$(O_{2}^{\bullet -})$$, NOX4 mainly produces hydrogen peroxide $$(H_{2}O_{2})$$. This is thanks to a special loop called the E-loop, which has a histidine residue that speeds up the conversion of superoxide to hydrogen peroxide right inside the enzyme.
The reaction equation for NOX4 can be represented as:
$$ \mathrm{NADPH} + 2\mathrm{O}_2 + \mathrm{H}^+ \to \mathrm{NADP}^+ + 2\mathrm{H}_2\mathrm{O}_2 $$
This equation shows how NOX4 efficiently generates hydrogen peroxide, with about 90 percent of its output being this form. The process involves two steps: first, oxygen gets one electron to become superoxide, then quickly grabs another electron and protons to form hydrogen peroxide. This rapid second step prevents superoxide from escaping, making NOX4 safer for cells since hydrogen peroxide diffuses easily and triggers specific signals without as much chaos.
Structurally, NOX4 also has an ATP-binding site in some locations, like mitochondria, allowing it to sense energy levels. When ATP is low, it ramps up ROS production, helping cells adapt. Imagine a cell running low on fuel; NOX4 notices and sends out hydrogen peroxide signals to shift metabolism, like switching to glycolysis in tough times.
Unique Features of NOX4
What really makes NOX4 stand out from its siblings? Let us break it down with some key points:
- Constitutive Activity: Unlike NOX1 or NOX2, which wait for signals like cytokines to assemble and activate, NOX4 is always ready to go. Its output is mostly controlled by how much of it cells make through gene expression.
- Primary Product is Hydrogen Peroxide: This is huge because hydrogen peroxide is less reactive than superoxide and acts as a signaling molecule. For example, it can activate pathways for cell growth or protection without causing immediate damage.
- Dependency on p22phox Only: No need for cytosolic helpers like p47phox or Rac. This simplicity means NOX4 works steadily in places like the endoplasmic reticulum or mitochondria.
- Varied Subcellular Locations: Depending on the cell, NOX4 hangs out in the nucleus, mitochondria, or even focal adhesions. In heart cells, mitochondrial NOX4 links to energy sensing, while nuclear versions might affect gene regulation.
- Role as an Oxygen Sensor: With a high affinity for oxygen, NOX4 adjusts ROS based on oxygen levels, helping cells respond to low oxygen environments, like in tumors or during strokes.
These features give NOX4 a dual personality: helpful in normal conditions but potentially harmful when overexpressed. For instance, in kidney cells, it helps regulate sodium channels for proper fluid balance, but too much can lead to fibrosis.
Regulation of NOX4 Activity
Controlling NOX4 is mostly about turning its gene on or off, but there are layers to it. Transcription factors like NF-κB, SMAD2/3, and HIF-1α boost its expression in response to stress, growth factors, or low oxygen. Take transforming growth factor-beta (TGF-β); it strongly upregulates NOX4 in blood vessels, promoting remodeling.
Post-translationally, things get interesting. Proteins like POLDIP2 enhance NOX4 activity by directing it to the cytoskeleton, aiding cell movement. In mitochondria, the ATP-binding motif lets NOX4 respond to energy dips, increasing ROS to reprogram metabolism. MicroRNAs and histone changes also fine-tune levels; for example, certain miRNAs dial down NOX4 in healthy cells to prevent overload.
External factors play a role too. Angiotensin II, a hormone linked to high blood pressure, cranks up NOX4 in vessels. Shear stress from blood flow or endoplasmic reticulum stress can do the same. On the flip side, antioxidants or PPAR-gamma activators lower it. This regulation ensures NOX4 adapts to needs, but in diseases, it often spirals out of control.
Physiological Roles of NOX4
In a healthy body, NOX4 wears many hats. It supports angiogenesis, the growth of new blood vessels, by producing hydrogen peroxide that signals cells to migrate and form tubes. This is crucial for wound healing or pregnancy.
In the kidneys, NOX4 helps fine-tune ion transport. For example, it mediates how prorenin activates sodium channels in duct cells, keeping electrolyte balance. It also senses flow in tubules to adjust potassium currents, preventing buildup during urine formation.
The heart benefits too. NOX4 in endothelial cells promotes relaxation of blood vessels, lowering pressure. In mitochondria, it acts as an energy watchdog, boosting ROS when ATP drops to shift cells toward survival modes.
Even in the brain, NOX4 contributes to neuron signaling and protection under mild stress. Overall, it maintains redox balance, ensuring ROS serve as helpful messengers rather than foes.
NOX4 in Cardiovascular Diseases
The heart and vessels are where NOX4 shines and sometimes stumbles. Its dual role means it can protect or harm, depending on the situation.
In hypertension, studies show mixed results. Some find NOX4 helps by enhancing vasodilation through hydrogen peroxide, stabilizing blood pressure. Others link its upregulation to stiffer arteries and higher pressure, especially with angiotensin II involvement.
Atherosclerosis sees NOX4 as more of a guardian. It stabilizes plaques and reduces inflammation, unlike NOX1 or NOX2. Knockout mice without NOX4 sometimes develop worse lesions, suggesting its hydrogen peroxide curbs excessive immune responses.
For ischemia-reperfusion injury, like after a heart attack, NOX4 often worsens damage by fueling mitochondrial ROS and cell death. Yet, in some models, mild activity protects by preconditioning cells.
Heart failure and fibrosis are complex. Elevated NOX4 drives fibroblast growth, leading to scar tissue buildup. But cardiac-specific deletions yield conflicting data; sometimes it helps, sometimes not, highlighting context matters.
Let us organize this with a table comparing NOX4 roles in cardiovascular conditions:
| Condition | Role of NOX4 | Key Mechanisms | Examples from Studies |
|---|---|---|---|
| Hypertension | Debated; protective in some, contributory in others | Enhances vasodilation via H2O2; interacts with angiotensin II signaling | Angiotensin II-infused mice show increased aortic H2O2 |
| Atherosclerosis | Generally protective | Stabilizes plaques, reduces inflammation | Knockout mice have larger lesions in high-fat diets |
| Ischemia-Reperfusion | Often detrimental | Induces mitochondrial dysfunction and eNOS uncoupling | Reduced infarct size in NOX4-deleted hearts |
| Heart Failure | Contributes to hypertrophy and fibrosis | Activates NFAT via HDAC4 oxidation | Upregulated in pressure overload models |
| Aortic Aneurysms | Critical in formation | Promotes eNOS uncoupling and H4B deficiency | Deletion prevents aneurysms in genetic models |
| Cardiac Arrhythmias | Promotes arrhythmogenesis | Activates CaMKII through ROS | Overexpression leads to atrial fibrillation in models |
This table shows how NOX4 interacts with other systems, like mitochondria or eNOS, amplifying effects in diseases.
NOX4 in Cancer
Cancer cells love NOX4 because it helps them thrive in harsh environments. It is overexpressed in many types, like pancreatic, lung, renal, and thyroid cancers, fueling growth and spread.
One way is through metabolic reprogramming. In mitochondria, NOX4 senses low ATP and boosts ROS to favor glycolysis over normal respiration, a hallmark of cancer called the Warburg effect. This gives tumors energy even in low-oxygen spots.
NOX4 also promotes survival signals. Hydrogen peroxide from NOX4 activates pathways like MAPK or AKT, pushing cells to divide and resist death. In gastric cancer, it drives proliferation while blocking apoptosis.
Migration and invasion get a boost too. NOX4 helps cancer cells move by remodeling the cytoskeleton and activating matrix-degrading enzymes. For non-small cell lung cancer, high NOX4 links to metastasis and poor prognosis.
Drug resistance is another trick. By altering metabolism, NOX4 makes cells tougher against chemo, like in pancreatic tumors where it shields against gemcitabine.
Pan-cancer analyses show NOX4 as a biomarker; high levels predict worse outcomes in most tumors. But in some, like certain brain cancers, it might suppress growth, showing context dependency.
Here are some mechanisms in bullet form:
- Proliferation: Activates MEK1/2-ERK1/2 pathway in colorectal cancer, leading to faster cell division.
- Apoptosis Resistance: Generates ROS that inhibit death signals, as in brain endothelial cells under stress.
- Angiogenesis: Promotes new vessel formation for tumor nutrition, via VEGF signaling.
- Metabolic Shift: Low ATP activation increases glycolysis, aiding survival in hypoxic cores.
Examples abound: In pancreatic cancer, NOX4 knockout slows tumor growth in mice, suggesting it as a target.
To visualize, consider this table on NOX4 in specific cancers:
| Cancer Type | Expression Level | Main Roles | Mechanisms Involved | Potential Therapies |
|---|---|---|---|---|
| Pancreatic | High | Promotes progression, drug resistance | ATP sensing, glycolysis shift | NOX4 inhibitors like GKT137831 |
| Lung (Non-Small Cell) | High | Enhances proliferation, metastasis | ROS-mediated AKT activation | Targeted knockdown |
| Renal | High | Supports cell survival, angiogenesis | H2O2 signaling for VEGF | Combination with chemo |
| Thyroid | Variable | Contributes to invasion | MAPK pathway | Small molecule blockers |
| Colorectal | High | Drives tumor growth | MEK1/2-ERK1/2 | Metabolic disruptors |
| Gastric | High | Regulates apoptosis and growth | ROS generation | Antioxidant adjuncts |
This highlights NOX4 as a common thread in cancer biology.
NOX4 in Neurological Disorders
The brain is sensitive to ROS, and NOX4 plays a big part in disorders here. In ischemic stroke, NOX4 surges after blood flow stops, producing ROS that damage neurons and expand the infarct area. Deleting NOX4 in mice cuts damage by 75 percent, showing its harmful side.
Parkinson’s disease involves NOX4 too. In models using MPTP toxin, NOX4 in the hippocampus drives inflammation via cytokines, worsening neuron loss. It links to mitochondrial ROS, amplifying degeneration.
Fibrosis in the brain, like after injury, sees NOX4 promoting scar formation. Similar to other organs, it activates fibroblasts through TGF-β.
Alzheimer’s might involve NOX4, with ROS contributing to plaque buildup and tau tangles, though more research is needed.
Protective aspects exist; low-level NOX4 activity could shield neurons under mild stress by activating adaptive pathways.
A table for neurological roles:
| Disorder | Role of NOX4 | Key Mechanisms | Insights from Models |
|---|---|---|---|
| Ischemic Stroke | Detrimental, increases neuronal damage | Upregulation leads to ROS burst | 75% infarct reduction in knockouts |
| Parkinson’s Disease | Promotes inflammation and degeneration | NOX4-derived mtROS in hippocampus | MPTP models show cytokine involvement |
| Brain Fibrosis | Drives scar tissue formation | Activates fibroblasts via TGF-β | Post-injury upregulation |
| Neurodegenerative | Contributes to oxidative stress | Interactions with NOX2 in amyloid pathology | Potential in Alzheimer’s plaques |
These point to NOX4 as a target for brain protection.
NOX4 in Fibrosis and Other Conditions
Fibrosis, the excessive scarring in organs, often features NOX4. In lungs, it fuels idiopathic pulmonary fibrosis by making fibroblasts multiply and deposit matrix. Kidney fibrosis in diabetes links to NOX4-driven inflammation and cell death.
In renal diseases overall, NOX4 is the main isoform, regulating normal functions like ion channels but turning harmful in injury or hypertension. It activates pathways for apoptosis or survival, depending on the stage.
Other areas include angiogenesis in eyes or wounds, where NOX4 helps but can overdo it in tumors.
Therapeutic Potential: Targeting NOX4
With NOX4 implicated in so many issues, drugs targeting it are promising. Inhibitors like GKT137831 block NOX4 and NOX1, showing benefits in fibrosis, cancer, and heart models. It is in trials for diabetic kidney disease.
Specific blockers are in development, aiming for isoform selectivity to avoid side effects. Gene therapies or miRNAs could dial down expression.
Challenges remain: since NOX4 has protective roles, timing and dosing matter. Combining with antioxidants or metabolic drugs might work best.
Future looks bright, with NOX4 as a hub for redox-based treatments.
Conclusion
NOX4 is a remarkable enzyme, bridging normal cell function and disease. Its ability to produce hydrogen peroxide steadily makes it essential yet risky. From protecting hearts to fueling cancers, understanding NOX4 deepens our grasp of redox biology. As research advances, targeting it could revolutionize treatments for chronic diseases. It reminds us how small molecules like ROS shape our health in big ways.
Frequently Asked Questions
FAQ 1: What are the key differences between NOX4 and other NADPH oxidase isoforms?
Understanding the NADPH oxidase family is crucial for grasping how cells manage reactive oxygen species, which play roles in everything from immune responses to disease progression. NOX4 is one of seven isoforms in humans, including NOX1 through NOX5, and DUOX1 and DUOX2. While all these enzymes transfer electrons from NADPH to oxygen to produce ROS, they vary significantly in structure, activation, products, and tissue distribution.
For instance, NOX1, NOX2, and NOX3 typically require assembly with cytosolic subunits like p47phox or NOXO1 for activation, making them responsive to stimuli such as cytokines or pathogens. In contrast, NOX4 is constitutively active, meaning it produces ROS steadily without needing these extra triggers, and its activity is mainly regulated by how much protein is expressed through gene transcription.
Another major distinction lies in the type of ROS produced. Most isoforms, like NOX1 and NOX2, generate superoxide, a highly reactive molecule that can quickly cause cellular damage if not controlled. NOX4, however, primarily outputs hydrogen peroxide, which is less destructive and often acts as a signaling molecule in processes like cell growth and differentiation. This difference stems from NOX4‘s unique E-loop structure, which facilitates the rapid conversion of superoxide to hydrogen peroxide inside the enzyme.
NOX5 and the DUOX enzymes stand out with calcium-binding EF-hand domains, allowing them to respond to calcium signals, something NOX4 lacks. Tissue-wise, NOX2 is famous for its role in phagocytes for killing bacteria, while NOX4 is abundant in non-immune cells like those in the kidney, heart, and brain.
These variations mean each isoform has specialized functions. NOX3, for example, is mostly in the inner ear and aids in balance, whereas DUOX1 and DUOX2 are key in thyroid hormone production. In diseases, isoforms like NOX1 and NOX2 are often linked to excessive inflammation, but NOX4‘s steady hydrogen peroxide production can be protective in some contexts, like stabilizing blood vessel plaques, or harmful in others, such as promoting fibrosis. Researchers are exploring these differences to develop targeted therapies, as blocking one isoform without affecting others could minimize side effects.
To highlight these distinctions clearly, here is a comprehensive comparison table:
| Isoform | Primary ROS Product | Activation Mechanism | Key Tissue Distribution | Main Physiological Role | Disease Associations |
|---|---|---|---|---|---|
| NOX1 | Superoxide | Requires cytosolic subunits (NOXO1, NOXA1) | Colon, vascular smooth muscle | Cell signaling, angiogenesis | Hypertension, colorectal cancer |
| NOX2 | Superoxide | Requires assembly (p47phox, p67phox, Rac) | Phagocytes, brain | Immune defense, neuroinflammation | Chronic granulomatous disease, stroke |
| NOX3 | Superoxide | Needs NOXO1, sometimes p47phox | Inner ear, fetal tissues | Otolith formation in ear | Hearing loss, balance disorders |
| NOX4 | Hydrogen peroxide | Constitutively active, regulated by transcription | Kidney, heart, brain, vessels | Oxygen sensing, differentiation | Fibrosis, cancer, cardiovascular diseases |
| NOX5 | Superoxide | Calcium-dependent (EF-hands) | Lymphocytes, spleen, testis | Calcium signaling, apoptosis | Prostate cancer, cardiovascular issues |
| DUOX1 | Hydrogen peroxide | Calcium and peroxidase domain | Thyroid, airways, gut | Thyroid hormone, antimicrobial | Thyroid disorders, infections |
| DUOX2 | Hydrogen peroxide | Similar to DUOX1 | Thyroid, gastrointestinal tract | Hormone synthesis, mucosal defense | Congenital hypothyroidism |
This table underscores why NOX4 is unique and why targeting it specifically could revolutionize treatments for conditions where balanced ROS signaling is key.
FAQ 2: In which tissues is NOX4 most commonly expressed, and what does this mean for its functions?
NOX4 expression varies across the body, reflecting its versatile roles in maintaining cellular health and responding to stress. Originally identified in the kidney, where it helps regulate blood flow and oxygen sensing, NOX4 is now known to be present in a wide array of tissues. In the cardiovascular system, it’s highly abundant in endothelial cells lining blood vessels, cardiac myocytes, and vascular smooth muscle, contributing to vessel tone and protection against oxidative damage. For example, in the heart, NOX4 in mitochondria acts as an energy sensor, producing hydrogen peroxide to signal metabolic shifts during low ATP conditions.
Beyond the heart and vessels, NOX4 shows up in the brain, particularly in neurons and astrocytes, where it influences signaling and can exacerbate damage in strokes if overexpressed. In the lungs, it’s involved in epithelial cell function and fibrosis responses, while in the liver, it plays a part in metabolic regulation and fibrosis during diseases like non-alcoholic fatty liver disease. The pancreas expresses NOX4 too, linking it to insulin signaling and potential roles in diabetes complications. Even in bones, NOX4 in osteoclasts aids in bone resorption, and in the skin, it supports keratinocyte differentiation.
This broad distribution means NOX4 isn’t just a one-trick enzyme; it’s a key player in tissue-specific processes:
- Kidney and urinary system: High expression supports filtration and protects against hypertensive damage, but excess can lead to fibrosis.
- Nervous system: In the hippocampus and cortex, it modulates neurogenesis, with implications for aging and neurodegenerative conditions.
- Reproductive tissues: Found in ovaries and uterus, influencing fertility and hormone responses.
- Immune-related organs like spleen: Helps in lymphocyte function, tying into immune modulation.
- Cancer-prone sites such as breast, lung, and colon: Upregulated levels correlate with tumor growth, making it a potential marker for malignancy.
Overall, the widespread presence of NOX4 highlights its importance in redox balance across organs, but it also explains why dysregulation can contribute to diverse diseases, from metabolic syndromes to cancers.
FAQ 3: How do genetic variations in NOX4 influence disease risk and progression?
Genetic variations in NOX4 can subtly alter its function, potentially tipping the balance toward disease in susceptible individuals. Single nucleotide polymorphisms, or SNPs, are the most common changes, where a single DNA base pair differs among people. Some SNPs in the NOX4 gene might affect how much protein is produced or how efficiently it generates hydrogen peroxide. For instance, certain variants have been linked to altered ROS levels, which could exacerbate oxidative stress in conditions like cardiovascular diseases or cancers. In populations with high rates of hypertension, specific NOX4 polymorphisms might increase susceptibility by enhancing enzyme activity in blood vessels, leading to stiffer arteries over time.
In cancer, genetic alterations in NOX4 are less about mutations causing the disease outright and more about how variants influence tumor environments. Studies show that some NOX4 SNPs correlate with higher expression in tumors like renal cell carcinoma, promoting metabolic reprogramming that helps cancer cells survive in low-oxygen conditions. Similarly, in neurodegenerative diseases such as Alzheimer’s, elevated NOX4 due to genetic factors might amplify neuronal damage through excessive ROS, interacting with proteins like amyloid-beta to worsen plaque formation. Research in diabetic models also suggests that NOX4 variants could impair kidney function, accelerating nephropathy by boosting fibrosis signals.
While not all variations are harmful—some might even be protective by dampening overactive ROS production—their impact often depends on environmental factors like diet or stress. Large-scale genome-wide association studies are uncovering more links, but challenges remain in pinpointing causality. Future personalized medicine could screen for NOX4 variants to predict disease risk, guiding preventive strategies or targeted therapies.
FAQ 4: What can we learn from the evolutionary history of NOX4?
The story of NOX4‘s evolution offers fascinating insights into how life adapted to oxygen, a once-toxic element that became essential for complex organisms. NADPH oxidases likely emerged in ancient prokaryotes as a way to handle oxidative stress, with early forms resembling bacterial ferric reductases that shuttled electrons. In eukaryotes, the family diversified, with NOX4 appearing as a distinct isoform around the time of early metazoans, before the split between chordates and arthropods. Phylogenetic analyses show NOX4 branching from a common ancestor with NOX1-3, but it evolved unique features like constitutive activity and hydrogen peroxide production, possibly to support signaling in multicellular life.
In invertebrates, arthropod-specific variants like NOX4-art highlight independent evolutions, where gene duplications created specialized enzymes for roles in development or immunity. Vertebrates retained NOX4 for its reliability in non-phagocytic cells, contrasting with NOX2‘s immune-focused adaptations. This conservation across species—from sea urchins to humans—suggests NOX4 was crucial for oxygen sensing in emerging tissues like kidneys and hearts. Losses in some lineages, like certain insects, indicate redundancy or environmental adaptations.
Studying this history helps explain NOX4‘s dual roles today: protective in physiology but problematic in modern diseases influenced by lifestyle. It also guides research into why some animals resist oxidative damage better, potentially inspiring therapies.
FAQ 5: Could NOX4 serve as a diagnostic biomarker for various diseases?
NOX4 is emerging as a promising biomarker due to its consistent upregulation in diseased tissues, offering a window into oxidative stress levels. In cancers, elevated NOX4 in blood or tumor samples often signals aggressive progression; for example, in pancreatic or lung cancers, high levels correlate with poor survival, making it a potential blood test target for early detection. Similarly, in cardiovascular conditions, increased NOX4 in plasma reflects vascular damage, helping diagnose hypertension or atherosclerosis before symptoms worsen.
Its diagnostic value shines in pan-cancer analyses, where NOX4 expression patterns distinguish malignant from normal tissues across types like breast or colorectal cancer. Combined with immune markers, it could predict immunotherapy responses, as low NOX4 might indicate better T-cell infiltration.
Here’s a table summarizing NOX4‘s biomarker potential in key diseases:
| Disease Category | Biomarker Role | Detection Methods | Prognostic Implications | Limitations |
|---|---|---|---|---|
| Cancer (e.g., pancreatic, lung) | Upregulation indicates tumor growth | RT-PCR, immunohistochemistry | High levels predict poor survival | Variability across subtypes |
| Cardiovascular (hypertension, heart failure) | Elevated in serum for severity assessment | ELISA, Western blot | Correlates with fibrosis risk | Overlaps with other ROS sources |
| Neurological (stroke, Alzheimer’s) | Increased in brain tissue for damage extent | qPCR, imaging | Linked to neuronal loss | Hard to access in living patients |
| Metabolic (diabetes, fibrosis) | Marker of kidney/liver stress | Blood assays | Predicts complication onset | Influenced by diet |
| Immune-related (autoimmune diseases) | Reflects inflammation | Flow cytometry | Guides therapy response | Needs validation in large cohorts |
This positions NOX4 as a versatile tool, though more clinical trials are needed for standardization.
FAQ 6: What are the latest developments in NOX4 inhibitors and their clinical trials?
Efforts to target NOX4 with inhibitors are gaining momentum, as blocking its activity could curb excessive ROS in diseases without affecting beneficial isoforms. GKT137831, a dual NOX1/NOX4 inhibitor, has advanced furthest, showing promise in reducing fibrosis in diabetic kidney disease during phase II trials. Recent updates indicate it’s safe and improves outcomes in liver fibrosis models, with ongoing studies exploring its use in idiopathic pulmonary fibrosis.
Other compounds like GLX481304 selectively hit NOX2/NOX4, enhancing heart function post-ischemia in animal tests. Fulvene-5 and DPI are earlier-stage, but broad-spectrum, limiting specificity.
Key developments include:
- Phase I/II trials: GKT137831 completed safety checks; now testing in primary biliary cholangitis.
- Cancer applications: Inhibitors sensitize tumors to chemo by disrupting NOX4-driven resistance.
- Neurological focus: Potential in stroke, where NOX4 blockers reduce neuronal death.
- Challenges: Achieving isoform selectivity to avoid immune suppression.
Future trials may combine inhibitors with immunotherapies for better efficacy.
FAQ 7: How does NOX4 contribute to metabolic diseases like diabetes?
In metabolic diseases such as diabetes, NOX4 acts as a double-edged sword, influencing insulin signaling and tissue damage through ROS. In diabetic cardiomyopathy, upregulated NOX4 in cardiac cells boosts oxidative stress, leading to fibrosis and impaired heart function. Studies in diabetic mice show NOX4 deletion attenuates plaque formation without major side effects, highlighting its role in vascular complications.
It also reprograms metabolism in adipocytes and liver cells, promoting glycolysis over efficient energy use, exacerbating insulin resistance. In kidneys, NOX4-driven ROS accelerate nephropathy by activating inflammatory pathways.
FAQ 8: What are the promising directions for future research on NOX4?
Future research on NOX4 should prioritize isoform-specific inhibitors to dissect its roles without off-target effects, potentially revolutionizing treatments for fibrosis and cancer. Exploring its mitochondrial localization could reveal new links to aging and neurodegeneration, using advanced imaging to track real-time ROS dynamics.
Integrating genomics, like CRISPR editing of NOX4 variants, will clarify genetic influences on disease susceptibility. In immunotherapy, studying NOX4‘s impact on tumor microenvironments may uncover ways to boost T-cell responses.
Longitudinal studies in diverse populations are essential to validate NOX4 as a biomarker, paving the way for personalized medicine.
FAQ 9: What is the connection between NOX4 and neurodegenerative diseases like Alzheimer’s?
NOX4 links to Alzheimer’s through amplified oxidative stress in brain cells, where its upregulation in astrocytes promotes ferroptosis, a iron-dependent cell death exacerbating neuronal loss. In models, NOX4 interacts with amyloid-beta, heightening ROS that damage mitochondria and promote plaques.
In Parkinson’s, NOX4 in hippocampus drives inflammation via cytokines, worsening degeneration. Silencing NOX4 reduces damage in stroke models by 75%, suggesting therapeutic potential.
FAQ 10: How does NOX4 influence immune modulation in cancer?
NOX4 shapes cancer immunity by altering the tumor microenvironment, often suppressing effective responses. In breast cancer, NOX4 knockout enhances CD8+ T-cell activity, improving immunotherapy efficacy.
Here’s a table on its immune roles:
| Cancer Type | Immune Effect | Mechanisms | Therapeutic Potential |
|---|---|---|---|
| Breast | Reduces T-cell infiltration | Lowers CCL11/CCL5 chemokines | Combine with PD-1 inhibitors |
| Lung | Promotes suppressive macrophages | ROS-mediated signaling | Biomarker for response |
| Pancreatic | Enhances resistance | Metabolic shifts | Target for combo therapies |
| Glioblastoma | Activates TGF-beta | Immune evasion | Novel blockers |
| Renal | Correlates with checkpoints | High expression poor prognosis | Immunomodulatory drugs |
This underscores NOX4‘s role in tailoring immune-based treatments.
FAQ 11: How does NOX4 interact with other cellular pathways in health and disease?
NOX4, as a key member of the NADPH oxidase family, doesn’t operate in isolation but weaves into a complex network of cellular pathways, influencing everything from cell survival to inflammation. In healthy cells, NOX4’s production of hydrogen peroxide acts as a signaling molecule that can activate pathways like MAPK and AKT, which are crucial for cell growth and differentiation.
For instance, in endothelial cells, NOX4 interacts with the VEGF pathway to promote angiogenesis, helping form new blood vessels during tissue repair. This interaction is mediated by hydrogen peroxide oxidizing specific proteins, altering their activity to favor protective responses. However, when NOX4 levels rise due to stress, it can tip the balance toward harmful effects, such as in cancer where it boosts the PI3K/AKT pathway, enhancing tumor cell proliferation and resistance to therapies.
In cardiovascular contexts, NOX4 links with the RhoA/ROCK1 pathway, where it acts upstream to amplify signals leading to cell contraction and migration, which is vital for vascular remodeling but problematic in hypertension. Studies show that NOX4-generated ROS positively regulate RhoA, creating a feedback loop that sustains oxidative stress in smooth muscle cells. Similarly, in the heart, NOX4 interfaces with HDACs and transcription factors like NF-κB, modulating gene expression for inflammation or fibrosis. During hypoxia, NOX4 upregulation by HIF-1α enhances its own expression, forming a cycle that adapts cells to low oxygen but can exacerbate damage in strokes or heart attacks.
On the metabolic side, NOX4 senses ATP levels in mitochondria, binding directly to ATP to dial down its activity when energy is plentiful, thus preventing unnecessary ROS buildup. This ties into broader energy pathways, like shifting to glycolysis in cancer cells via the Warburg effect. In inflammatory settings, NOX4 converges with TLR4 signaling, promoting ferroptosis or apoptosis through ROS-dependent mechanisms. These intersections highlight NOX4’s role as a hub, where targeting it could disrupt multiple disease pathways without broad side effects, as seen in emerging inhibitors that block specific interactions like NOX4-GLI1 in gastric cancers.
FAQ 12: What is the role of NOX4 in aging and oxidative stress?
NOX4 plays a pivotal role in the aging process by contributing to chronic oxidative stress, particularly in tissues like the heart and vessels where it ramps up with age. As cells grow older, NOX4 expression increases in mitochondria, leading to higher ROS levels that damage proteins, lipids, and DNA, accelerating cellular senescence. This buildup is linked to age-related diseases, where NOX4 acts as a mediator of mitochondrial dysfunction, promoting inflammation and fibrosis. Interestingly, while excessive NOX4 is harmful, moderate activity might support adaptive responses, like in vascular smooth muscle cells where it maintains quiescence.
In animal models, knocking out NOX4 reduces mitochondrial ROS and improves outcomes in aging under high-fat diets, suggesting it mediates cardiovascular decline. Human studies echo this, with elevated NOX4 tied to arterial stiffening and atherosclerosis in older adults. Therapeutically, targeting NOX4 could mitigate aging effects, as inhibitors show promise in reducing oxidative damage without compromising essential signaling.
To illustrate NOX4’s involvement across systems, consider this detailed table:
| Aspect of Aging | NOX4’s Contribution | Key Mechanisms | Tissue-Specific Effects | Potential Interventions |
|---|---|---|---|---|
| Cardiovascular Decline | Upregulates mitochondrial ROS leading to dysfunction | ATP-sensing motif activates under low energy, oxidizing proteins like HDAC4 | Heart: Promotes fibrosis and failure; Vessels: Increases stiffness and plaque instability | NOX4 inhibitors like GKT137831 reduce inflammation and improve elasticity |
| Neurological Aging | Enhances neuronal damage via ROS burst | Interacts with TGF-β1 to boost inflammatory genes | Brain: Contributes to stroke damage and neurodegeneration | Genetic knockdown in models cuts infarct size by up to 75% |
| Metabolic Reprogramming | Shifts to glycolysis, worsening insulin resistance | Senses ATP dips to increase H2O2 signaling | Liver and muscle: Accelerates fatty liver and sarcopenia | Antioxidants or NOX4 deletion in mice prevents lipid accumulation |
| Cellular Senescence | Induces apoptosis and quiescence in smooth muscle | Oxidizes JmjD3, altering histone methylation | Vascular cells: Leads to reduced proliferation and repair | miRNA therapies to downregulate NOX4 expression |
| Overall Oxidative Stress | Major source of H2O2 in non-phagocytic cells | Constitutive activity unregulated by assembly | Multiple organs: Amplifies DNA damage and telomere shortening | Lifestyle changes like exercise modulate NOX4 levels for better aging outcomes |
FAQ 13: What environmental factors influence NOX4 expression and activity?
Environmental factors can significantly sway NOX4 levels, often through stress responses that alter gene transcription or protein stability. Pollutants like bisphenol A, found in plastics, have been shown to boost NOX4 in thyroid cells, promoting atypical growth and potentially linking everyday exposures to cancer risks. Hypoxia, common in high-altitude living or polluted areas, strongly induces NOX4 via HIF-1α, enhancing ROS for adaptation but risking chronic inflammation if prolonged.
Other influences include inflammatory triggers from infections or allergens, which upregulate NOX4 in airways, as seen in COPD where smoke exposure heightens its expression in smooth muscle, leading to ROS-mediated remodeling. Chemical stressors like angiotensin II in hypertension models mimic environmental pressures, cranking up NOX4 in vessels.
Key factors include:
- Air pollution and toxins: Particulates increase NOX4 in lungs and heart, exacerbating oxidative stress and fibrosis.
- Dietary components: High-fat diets elevate NOX4 in adipose tissue, tying into obesity-related diseases.
- Radiation and UV exposure: Boosts NOX4 in skin cells, contributing to premature aging.
- Infectious agents: Viruses like HCV induce NOX4 in liver, linking to chronic disease.
- Lifestyle stressors: Chronic stress hormones like cortisol may indirectly enhance NOX4 via inflammatory pathways.
FAQ 14: How is NOX4 involved in infectious diseases and immune responses?
In infectious diseases, NOX4 emerges as a modulator of immune responses, often amplifying inflammation through ROS production in non-immune cells. During viral infections like influenza, endothelial NOX4 can negatively regulate pathology by curbing excessive inflammation, as seen in mouse models where its absence worsens lung damage. This suggests NOX4 helps balance the immune storm, preventing overactivation that harms host tissues. In contrast, in chronic infections such as HCV, NOX4 in liver cells contributes to sustained ROS, fostering fibrosis and progression to severe disease.
Bacterial infections also involve NOX4, particularly in the gut where it’s upregulated in inflammatory bowel disease, protecting against acute flares but potentially fibrogenic in chronic cases. In neurological infections or sepsis, NOX4 in astrocytes drives ferroptosis, a form of cell death that exacerbates brain injury. Overall, NOX4’s role is context-dependent: protective in acute phases by aiding macrophage polarization toward anti-inflammatory states, but detrimental in prolonged infections by sustaining oxidative stress.
Therapeutic angles include using NOX4 inhibitors to dampen ROS in severe infections, potentially reducing organ damage without impairing bacterial clearance, as NOX4 isn’t central to phagocytic killing like NOX2.
FAQ 15: What do we know about the structural biology of NOX4?
The structural features of NOX4 set it apart in the NADPH oxidase family, enabling its unique hydrogen peroxide output. Comprising six transmembrane helices and a cytosolic dehydrogenase domain, NOX4 embeds in membranes with two heme groups orthogonally placed for electron transfer. The E-loop in its extracellular region contains a histidine that catalyzes superoxide dismutation to hydrogen peroxide, preventing free radical release.
Crystal structures reveal similarities with NOX2 but highlight NOX4’s lack of regulatory domains, explaining constitutive activity. It heterodimerizes with p22phox for stability, without needing cytosolic activators. Mutational studies show key motifs like the ATP-binding site in mitochondrial forms, allowing energy sensing.
Here’s a structured table on NOX4’s architecture:
| Structural Component | Description | Functional Role | Comparison to Other NOXs | Research Insights |
|---|---|---|---|---|
| Transmembrane Domain | Six helices spanning the membrane | Houses hemes for electron shuttling | Similar to NOX1-3, but no activation loops | Mutations here alter ROS output |
| Dehydrogenase Domain | N-terminal with NADPH/FAD binding | Electron donor site | Lacks EF-hands unlike NOX5 | Crystal models show ATP inhibition site |
| E-Loop | Extracellular histidine-rich loop | Dismutates superoxide to H2O2 | Unique to NOX4 for primary product | Explains milder signaling ROS |
| p22phox Interface | Binding site for stabilizer protein | Forms active heterodimer | Shared with NOX1-4 | Essential for folding and activity |
| Mitochondrial Motifs | ATP-binding sequence | Energy sensing in organelles | Not in cytosolic isoforms | Links to metabolic diseases |
FAQ 16: What animal models are used to study NOX4 and its functions?
Animal models provide invaluable insights into NOX4’s roles, with mice being the go-to due to genetic manipulability. Knockout mice lacking NOX4 globally or tissue-specifically, like in podocytes, reveal its protective effects in diabetic nephropathy, where deletion reduces kidney damage. In heart models, NOX4-deficient mice show less fibrosis post-injury, highlighting its fibrogenic potential.
Other models include transgenic overexpressors, simulating disease states like hypertension, where elevated NOX4 accelerates vascular stiffening. Rat models complement this, especially for liver regeneration studies where NOX4 deletion speeds healing after partial hepatectomy.
Common models encompass:
- Cardiac pressure overload: NOX4 knockouts mitigate mitochondrial stress and dysfunction.
- Ventilator-induced lung injury: Inhibiting NOX4 lessens inflammation and ROS.
- Alzheimer’s simulations: NOX4 modulation affects memory deficits via miR-204.
- Intravascular hemolysis AKI: Deletion protects kidneys from oxidative burst.
- Obesity and metabolic syndrome: High-fat diet models show NOX4’s sex-specific effects on insulin sensitivity.
FAQ 17: What is NOX4’s role in reproductive health and related disorders?
In reproductive health, NOX4 influences fertility and pregnancy outcomes through ROS signaling in ovarian and placental cells. In polycystic ovary syndrome models, NOX4 deficiency improves granulosa cell viability by curbing apoptosis and autophagy triggered by hormones like DHEA, suggesting it mediates oxidative damage in follicles. During pregnancy, maternal obesity links to placental NOX4 upregulation, causing senescence and impairing fetal development via exosomal transfer of oxidative stress.
In male reproduction, NOX4 in testicular cells regulates spermatogenesis, with imbalances tied to infertility. Its presence in granulosa cells responds to gonadotropins, producing H2O2 for ovulation signals, but excess can disrupt cycles.
Overall, NOX4’s dual nature means moderate levels support reproductive processes, while dysregulation from environmental or metabolic factors contributes to disorders like endometriosis or preeclampsia.
FAQ 18: How do dietary factors influence NOX4 activity?
Diet profoundly affects NOX4, with high-fat intakes often elevating its expression and contributing to metabolic woes. Low-sodium diets in NOX4-deficient models lower blood pressure more sharply, indicating NOX4 helps maintain vascular tone under restriction. Antioxidants from plants, like those in berries, can inhibit NOX4, reducing ROS in adipose tissue during obesity.
High-sugar diets mimic this, upregulating NOX4 in beta cells and fueling inflammation. Conversely, caloric restriction might dampen NOX4, promoting longevity.
This table details dietary impacts:
| Dietary Factor | Effect on NOX4 | Mechanisms | Health Implications | Examples from Studies |
|---|---|---|---|---|
| High-Fat Diet | Increases expression and activity | Enhances ROS in liver and muscle | Worsens insulin resistance, sex-specific in males | Mice show reduced adiposity with NOX4 knockout |
| Low-Sodium Intake | Modulates blood pressure response | Alters renal NOX4 signaling | Protects against hypertension | Knockouts exhibit pronounced BP drop |
| Antioxidants (e.g., Apocynin) | Inhibits activity | Scavenges ROS, blocks enzyme | Reduces obesity inflammation | Lowers triglycerides in males |
| High-Sugar/Overnutrition | Upregulates in beta cells | Activates inflammasome | Promotes diabetes complications | Sustains IL-1β in pancreatic models |
| Caloric Restriction | Potentially decreases | Via SIRT1 pathways | Improves metabolic health | Links to reduced fibrosis in aging |
FAQ 19: What is the connection between NOX4 and exercise physiology?
Exercise dynamically engages NOX4 in muscles and heart, where it facilitates adaptive responses like improved metabolism and antioxidant defenses. Acute bouts increase NOX4 expression, promoting H2O2 signals that enhance glucose and fat oxidation, boosting endurance. In skeletal muscle, NOX4 deletion blunts these benefits, leading to oxidative stress and insulin resistance with age.
Cardiac NOX4 supports exercise-induced Nrf2 activation, essential for handling stress and preventing damage. However, excessive NOX4 might hinder fiber switching in some contexts.
Connections include:
- Metabolic adaptation: NOX4-derived ROS activate pathways for fuel use during workouts.
- Antioxidant response: Upregulates defenses against exercise-induced ROS.
- Performance enhancement: Loss of NOX4 reduces capacity and vascular benefits.
- Aging effects: Maintains muscle health against sarcopenia.
- Therapeutic potential: Modulating NOX4 could optimize training outcomes.
FAQ 20: What are the emerging therapies targeting NOX4 for various diseases?
Emerging therapies focus on NOX4 as a biomarker and target, especially in cancers where its inhibition sensitizes tumors to TKIs and immunotherapy by curbing ROS-driven resistance. Compounds like GKT137831, in trials for fibrosis and diabetic complications, block NOX4 to reduce inflammation and oxidative damage. In cardiomyopathy, NOX4 inhibitors aim to halt remodeling and apoptosis, with potential in Duchenne models.
For ocular hypertension, GLX351322 alleviates glaucoma by suppressing glial activation. Pan-cancer strategies highlight NOX4’s prognostic value, guiding personalized treatments. Challenges include selectivity to avoid disrupting protective roles, but combinations with antioxidants show promise.
Future directions involve CRISPR for precise editing and natural compounds for milder modulation.
Acknowledgment
The Examsmeta.com website expresses its gratitude to the scientific community and various reputable online resources that have significantly contributed to the development of the article “Unraveling the Mysteries of NOX4: The Unique NADPH Oxidase Isoform in Health and Disease.” The comprehensive insights and data provided by these platforms were instrumental in ensuring the article’s depth and accuracy.
Specifically, acknowledges PubMed (pubmed.ncbi.nlm.nih.gov) for its extensive database of peer-reviewed studies, which offered critical research papers on NOX4’s structure, function, and disease implications. ScienceDirect (www.sciencedirect.com) provided access to high-quality articles detailing NOX4’s roles in cardiovascular and metabolic disorders, enriching our understanding of its clinical relevance.
Additionally, Nature (www.nature.com) contributed valuable reviews and studies on NOX4’s evolutionary biology and therapeutic potential, enhancing the article’s scientific rigor. These resources collectively enabled a thorough exploration of NOX4’s multifaceted roles, and we are thankful for their contributions to advancing knowledge in this field.

