Oxidative phosphorylation stands as one of the most vital processes in biology, serving as the primary way our cells generate the energy needed to sustain life. This intricate mechanism takes place within the mitochondria, often called the powerhouses of the cell, where it converts the energy from food into usable forms like ATP. Without it, complex life as we know it wouldn’t exist, as it allows aerobic organisms to extract far more energy from nutrients compared to simpler processes.
Imagine your body as a bustling city—oxidative phosphorylation is the efficient power grid that keeps everything running smoothly, from muscle contractions to brain activity. It’s a series of redox reactions that not only produce ATP but also highlight the evolutionary leap from anaerobic to aerobic life, enabling diverse ecosystems on Earth.
Table of Contents
Oxidative phosphorylation involves the electron transport chain (ETC), a lineup of protein complexes that shuttle electrons from molecules like NADH and FADH2 to oxygen, the ultimate electron acceptor. This flow releases energy, which pumps protons across the mitochondrial membrane, creating a gradient that drives ATP synthesis. It’s fascinating how this process ties into everyday health—disruptions can lead to fatigue, neurological issues, or even severe diseases.
Researchers continue to uncover its nuances, revealing connections to aging, cancer, and metabolic disorders. By understanding oxidative phosphorylation, we gain insights into why oxygen is essential for most lifeforms and how ancient evolutionary events shaped modern biology.
Fundamentals of Oxidative Phosphorylation
To grasp oxidative phosphorylation, it’s essential to start with a few key concepts that form its foundation. First, consider electronegativity, which measures how strongly an atom pulls electrons toward itself. Oxygen ranks high in electronegativity, second only to fluorine, making it an ideal final electron acceptor in this process. Its abundance in the atmosphere further cements its role, allowing cells to efficiently harness energy without relying on rarer elements.
Next, the sources of electron carriers like NADH and FADH2 are crucial. These molecules arise from catabolic pathways such as glycolysis, the citric acid cycle, and fatty acid oxidation. NADH, for instance, forms when NAD+ picks up electrons during the breakdown of glucose, carrying high-energy electrons to the mitochondria. Similarly, FADH2 emerges from reactions in the citric acid cycle, providing another entry point for electrons into the ETC. These carriers act like delivery trucks, transporting energy from food breakdown to the site of ATP production.
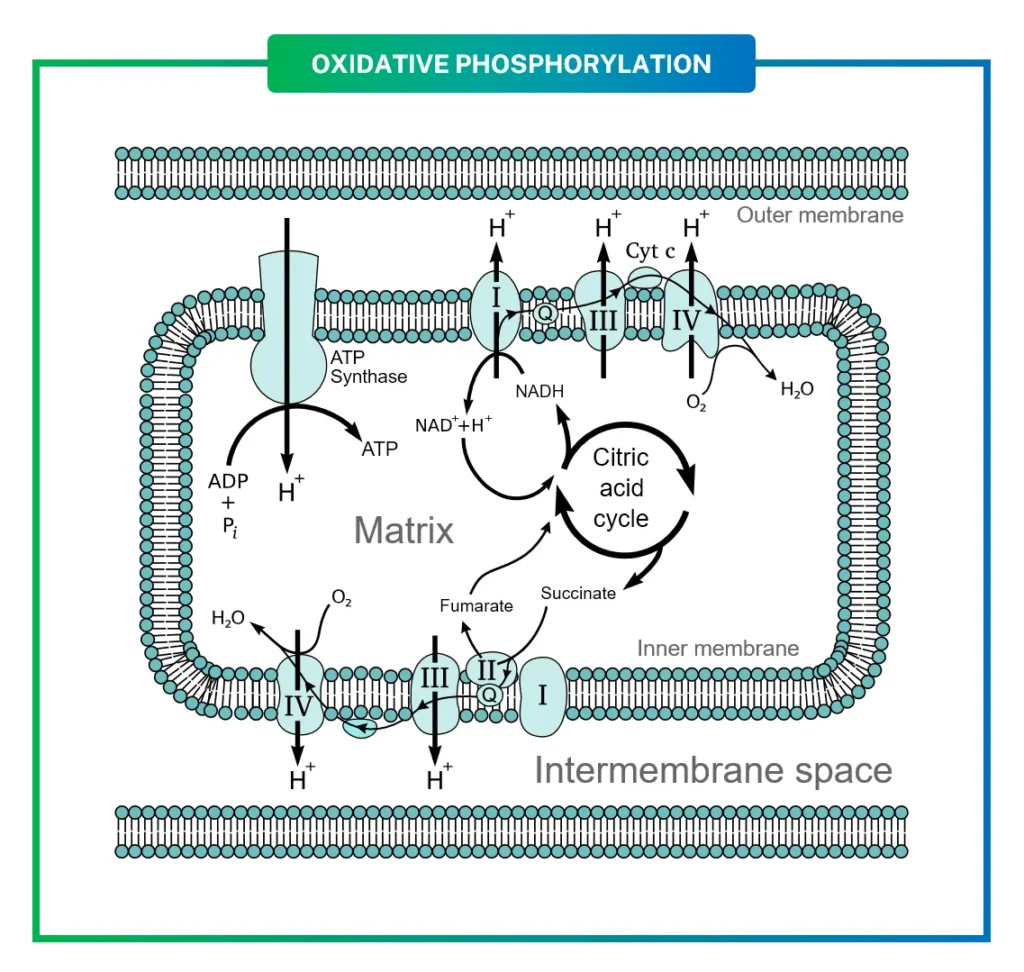
The structure of the mitochondrion itself is perfectly suited for this task. With its double membrane—an outer one that’s permeable and an inner one folded into cristae—the mitochondrion maximizes surface area for the ETC. The inner membrane hosts the protein complexes, while the matrix inside contains enzymes, DNA, and ribosomes. The space between membranes, the intermembrane space, becomes a reservoir for protons, building the electrochemical gradient that powers ATP synthesis. This setup isn’t just efficient; it’s a testament to evolutionary refinement, allowing eukaryotic cells to produce energy on a scale far beyond prokaryotes.
- Electronegativity in Action: Oxygen’s pull ensures electrons move downhill in energy terms, releasing usable power step by step.
- Electron Carrier Production: In a single glucose molecule’s breakdown, glycolysis yields 2 NADH, while the citric acid cycle adds 6 more NADH and 2 FADH2.
- Mitochondrial Anatomy Example: The cristae folds can increase the inner membrane’s area by up to five times, boosting ATP output in high-energy tissues like heart muscle.
Additional insights reveal that mitochondrial dynamics, such as fusion and fission, help maintain this structure under stress, ensuring optimal function during exercise or fasting.
Evolution of Oxidative Phosphorylation
The story of oxidative phosphorylation is deeply intertwined with the evolution of life on Earth. Billions of years ago, when oxygen levels rose during the Great Oxidation Event around 2.4 billion years ago, early cells adapted by incorporating oxygen into their metabolism. This shift from anaerobic fermentation to aerobic respiration marked a pivotal moment, allowing for a massive increase in energy yield and supporting the development of multicellular organisms.
Mitochondria themselves originated from an ancient symbiotic event, where a prokaryotic cell engulfed an aerobic bacterium over 1.45 billion years ago, leading to the eukaryotic cell we see today. This endosymbiosis provided the host cell with efficient energy production via the ETC, while the bacterium gained protection and nutrients. Over time, genes from the mitochondrial genome transferred to the nucleus, creating a hybrid system reliant on both nuclear and mitochondrial DNA.
In eukaryotes, oxidative phosphorylation became ubiquitous, powering everything from single-celled algae to complex animals. Compared to prokaryotes, which might use alternative electron acceptors like nitrate, eukaryotic systems are more specialized for oxygen, reflecting adaptations to an oxygen-rich world. This evolution also explains why some anaerobic eukaryotes, like certain parasites, have modified mitochondria called mitosomes that lack a full ETC.
- Key Evolutionary Milestones: The rise of cyanobacteria produced oxygen, setting the stage for aerobic respiration.
- Gene Transfer Example: About 90% of mitochondrial proteins are now encoded by nuclear DNA, with only a few genes remaining in mitochondrial DNA for quick response to energy needs.
- Adaptations in Extremophiles: Some deep-sea bacteria use hydrogen sulfide in their ETC, showing diverse evolutionary paths.
Recent studies suggest that variations in ETC efficiency influenced speciation, with more efficient systems enabling larger body sizes and complex behaviors in animals.
Issues of Concern in Oxidative Phosphorylation Research
Despite its efficiency, oxidative phosphorylation isn’t without challenges, and several areas remain underexplored. One major issue is the secondary regulation of the ETC, such as through reversible phosphorylation of protein complexes. This could fine-tune energy production in response to cellular signals, but how it interacts with pathways like insulin signaling is still unclear.
Another concern links mitochondrial function to neuroplasticity—the brain’s ability to form new connections. Dysfunctional mitochondria might impair synaptic plasticity, contributing to cognitive decline in aging. Exploring these ties could reveal how mitochondrial defects exacerbate neurological disorders, potentially leading to targeted therapies.
By delving deeper into these mechanisms, scientists aim to unravel the pathogenesis of diseases where energy production falters. For instance, understanding proton leaks or uncoupling proteins might explain variations in metabolic rates among individuals.
- Underexplored Regulations: Phosphorylation might activate or inhibit complexes during stress, like in hypoxia.
- Neuroplasticity Link Example: In Alzheimer’s models, impaired ETC correlates with reduced synaptic strength.
- Therapeutic Potential: Modulating these regulations could treat conditions like Parkinson’s, where mitochondrial toxins play a role.
Ongoing research emphasizes the need for better models to study these interactions, highlighting gaps in our knowledge of mitochondrial signaling networks.
Cellular Level Insights into Oxidative Phosphorylation
On a cellular scale, oxidative phosphorylation couples the exergonic transfer of electrons to the endergonic synthesis of ATP, ensuring energy balance. This process yields about 30-32 ATP per glucose molecule, dwarfing glycolysis’s mere 2 ATP. The efficiency stems from chemiosmosis, where proton pumping creates a gradient across the inner membrane, known as the proton motive force.
This force not only drives ATP production but also maintains cellular homeostasis. In muscle cells during exercise, for example, increased demand ramps up the ETC, preventing lactic acid buildup seen in anaerobic conditions.
The first law of thermodynamics holds here—the energy from electron transfers equals that captured in ATP bonds. Disruptions, like in hypoxic tissues, force cells to rely on less efficient pathways, leading to fatigue.
- Energy Coupling Example: Catabolic reactions fuel anabolic ones, like protein synthesis.
- Proton Motive Force Details: It combines pH differences and membrane potential for robust energy harnessing.
- Cellular Adaptations: Cancer cells often shift to glycolysis (Warburg effect) to avoid ETC dependency.
Further, cellular compartments ensure isolation—matrix enzymes handle citric cycle intermediates, while the membrane sequesters the ETC.
Molecular Level Breakdown
Diving into the molecules, the ETC comprises four main complexes (I-IV) plus ATP synthase (V), each with unique structures aiding electron flow. Complexes I, III, and IV pump protons, while II does not, reflecting different energy releases.
Mobile carriers like coenzyme Q10 (ubiquinone) and cytochrome c shuttle electrons between complexes. Coenzyme Q10, with its lipid-soluble tail, diffuses in the membrane, carrying two electrons as ubiquinol. Cytochrome c, water-soluble, transfers single electrons via its heme iron.
Genes for these components span mitochondrial and nuclear DNA, requiring coordinated expression. Mutations here can disrupt assembly, leading to inefficiencies.
- Prosthetic Groups: Iron-sulfur clusters in complexes facilitate redox reactions.
- Carrier Mobility Example: Cytochrome c’s detachment in apoptosis signals cell death.
- Genetic Dualism: Mitochondrial DNA encodes 13 ETC proteins, prone to mutations due to ROS exposure.
Molecular studies show how antioxidants like vitamin E protect these structures from oxidative damage.
Here’s a detailed table summarizing the ETC complexes:
| Complex | Common Name | Main Function | Protons Pumped | Key Components and Carriers |
|---|---|---|---|---|
| I | NADH Dehydrogenase | Oxidizes NADH, reduces ubiquinone | 4 | FMN, Fe-S clusters, ubiquinone |
| II | Succinate Dehydrogenase | Oxidizes FADH2, reduces ubiquinone | 0 | FAD, Fe-S clusters, links to citric cycle |
| III | Cytochrome c Reductase | Transfers electrons from ubiquinol to cytochrome c | 4 | Cytochromes b and c1, Fe-S, Q cycle |
| IV | Cytochrome c Oxidase | Reduces oxygen to water using cytochrome c | 4 | Heme a, copper ions, oxygen binding |
| V | ATP Synthase | Synthesizes ATP from proton flow | N/A (uses) | F0 channel, F1 catalytic head, rotary mechanism |
This table illustrates the sequential nature and contributions to the proton gradient.
Mechanism of Electron Transfer and ATP Synthesis
The mechanism begins with Complex I, where NADH donates electrons to FMN, then through Fe-S clusters to ubiquinone, pumping 4 protons.
The reaction: $$\text{NADH} + \text{Q} + 5\text{H}^+{\text{matrix}} \rightarrow$$ $$\text{NAD}^+ + \text{QH}_2 + 4\text{H}^+{\text{intermembrane}}$$.
Complex II handles FADH2 from succinate oxidation, transferring electrons to ubiquinone without proton pumping, explaining lower ATP from FADH2 (1.5 vs. 2.5 from NADH).
Electrons converge at Complex III via the Q cycle, where ubiquinol reduces cytochrome c, pumping 4 protons. This dimeric complex ensures efficient single-electron transfers.
In Complex IV, cytochrome c delivers electrons to reduce oxygen: $$4\text{Cyt c}^{2+} + \text{O}2 + 8\text{H}^+{\text{matrix}} \rightarrow$$ $$4\text{Cyt c}^{3+} + 2\text{H}2\text{O} + 4\text{H}^+{\text{intermembrane}}$$.
Finally, Complex V uses the gradient for ATP: Protons flow through F0, rotating F1 to bind ADP + Pi. Overall equation: $$\text{NADH} + \frac{1}{2}\text{O}_2 + \text{H}^+ + \text{ADP} + \text{P}_i \rightarrow$$ $$\text{NAD}^+ + \text{H}_2\text{O} + \text{ATP}$$.
- Q Cycle Details: Prevents radical formation by staggered electron release.
- Rotary Mechanism Example: Like a waterwheel, proton flow spins the gamma subunit 360 degrees for three ATP.
Electronegativity increases along the chain, driving spontaneous flow.
Comparison with Anaerobic Respiration
While oxidative phosphorylation thrives on oxygen, anaerobic respiration occurs without it, yielding far less ATP—only 2 per glucose via fermentation, versus 30-32 in aerobics. In anaerobes like yeast, pyruvate ferments to ethanol, regenerating NAD+ but wasting potential energy.
Aerobic processes are slower but more efficient, suitable for sustained activity, while anaerobic is rapid for bursts, like sprinting where muscles produce lactate.
- ATP Yield Breakdown: Aerobic: 2 from glycolysis, 2 from citric cycle, 26-28 from ETC; Anaerobic: 2 from glycolysis only.
- Organism Examples: Bacteria like Clostridium use anaerobic for survival in oxygen-free environments.
- Human Applications: During intense exercise, cells switch to anaerobic, causing muscle burn from lactic acid.
This comparison underscores why aerobic evolution was transformative.
Pathophysiology and Related Disorders
Pathophysiological issues arise when the ETC falters, often from toxins or mutations. Cyanide and carbon monoxide bind Complex IV, halting ATP production and causing rapid death. Drugs like amobarbital inhibit Complex I, reducing energy in sedated states.
Oxidative stress from ROS, byproducts of the ETC, damages DNA and proteins, accelerating aging and diseases like cancer. Mitochondrial myopathies, such as MELAS from MT-ND1 mutations, cause strokes and weakness due to impaired Complex I. MERRF involves ragged muscle fibers and epilepsy from multiple gene defects.
Other disorders include Leigh syndrome, affecting infants with brain lesions from ETC mutations, and Kearns-Sayre syndrome with eye and heart issues.
- ROS Effects: Superoxide from Complex I/III leads to cellular senescence.
- Toxin Examples: Rotenone blocks Complex I, used as pesticide but linked to Parkinson’s.
- Disease Symptoms: Fatigue, lactic acidosis common in OXPHOS defects.
Environmental toxins like heavy metals exacerbate these issues.
Here’s a table of mitochondrial diseases linked to OXPHOS:
| Disease | Affected Component | Genetic Cause | Main Symptoms | Prevalence |
|---|---|---|---|---|
| MELAS | Complex I | Mitochondrial DNA mutations | Strokes, lactic acidosis, encephalopathy | Rare, 1:4000 |
| MERRF | Multiple Complexes | mtDNA tRNA mutations | Myoclonus, epilepsy, muscle weakness | Very rare |
| Leigh Syndrome | Complexes I, IV | Nuclear/mtDNA mutations | Developmental delay, brain lesions | 1:40,000 |
| Kearns-Sayre | Multiple | Large mtDNA deletions | Eye muscle paralysis, heart block | Rare |
| NARP | ATP Synthase | mtDNA mutations | Neuropathy, ataxia, retinitis | Very rare |
This table highlights the diversity of impacts from OXPHOS dysfunction.
Recent Advances in Electron Transport Chain Research
As of 2025, research has unveiled exciting discoveries, like rhodoquinone in mammalian mitochondria, a second electron carrier challenging traditional views and potentially aiding ischemia treatments. This metabolite could bypass parts of the ETC under low oxygen, offering new therapeutic avenues.
Studies show mitochondria support T-cell proliferation and memory in immunity, with ETC remodeling influencing inflammasome activation. In aging, targeting ETC for longevity—via mild inhibition—extends lifespan in models, linking to drugs like rapamycin.
- Rhodoquinone Impact: Enhances flexibility in electron transport during stress.
- Immune Connections Example: Boosted ETC in T-cells improves vaccine responses.
- Longevity Strategies: Partial ETC sabotage triggers protective responses like mitophagy.
Advances in synthetic biology aim to engineer ETC localization for custom energy systems.
Clinical Significance and Applications
Clinically, oxidative phosphorylation’s role extends to therapies and diagnostics. Uncoupling proteins like thermogenin in brown fat generate heat for newborns, preventing hypothermia by dissipating the proton gradient.
In cancer, targeting OXPHOS inhibitors shows promise, as many tumors rely on it despite the Warburg effect, improving outcomes in resistant cases. For metabolic diseases, enhancing ETC function via exercise or supplements like coenzyme Q10 aids conditions like heart failure.
Diagnostics use biomarkers of mitochondrial dysfunction, like elevated lactate, for early detection of myopathies.
- Therapeutic Uncoupling: Drugs mimicking thermogenin combat obesity by burning fat.
- Cancer Application Example: IACS-010759 inhibits Complex I, starving tumor cells.
- Drug Toxicity Screening: Assessing ETC effects prevents side effects in new medications.
Overall, harnessing oxidative phosphorylation could revolutionize treatments for energy-related disorders, from diabetes to neurodegeneration.
Frequently Asked Questions
FAQ 1: What is oxidative phosphorylation and how does it contribute to cellular energy production?
Oxidative phosphorylation is a fundamental biochemical process that occurs in the mitochondria of eukaryotic cells, serving as the final stage of cellular respiration where the bulk of ATP, the cell’s energy currency, is generated. This process couples the oxidation of nutrients with the phosphorylation of ADP to form ATP, harnessing energy from electrons transferred through a series of protein complexes. It begins with electron carriers like NADH and FADH2, produced from earlier metabolic pathways such as glycolysis and the citric acid cycle, donating electrons to the electron transport chain embedded in the inner mitochondrial membrane.
As electrons flow through the chain, they release energy that pumps protons from the mitochondrial matrix into the intermembrane space, creating an electrochemical gradient known as the proton motive force. This gradient drives protons back into the matrix through ATP synthase, a rotary enzyme that catalyzes ATP synthesis. The overall reaction can be summarized as $$\text{NADH} + \frac{1}{2}\text{O}_2 + \text{H}^+ + \text{ADP} + \text{P}_i \rightarrow $$ $$\text{NAD}^+ + \text{H}_2\text{O} + \text{ATP}$$, highlighting oxygen’s role as the final electron acceptor. Without this step, cells would rely solely on less efficient pathways, yielding far fewer ATP molecules.
The significance of oxidative phosphorylation extends beyond mere energy production; it maintains cellular homeostasis by regulating redox balance and influencing signaling pathways. In high-energy-demand tissues like the heart and brain, efficient oxidative phosphorylation ensures sustained function, while impairments can lead to metabolic imbalances. Evolutionary, this process marked a shift from primitive anaerobic metabolism, enabling complex life forms to thrive in oxygen-rich environments by extracting up to 30-32 ATP per glucose molecule, compared to just 2 from glycolysis alone.
FAQ 2: What are the key complexes involved in the electron transport chain during oxidative phosphorylation?
| Complex | Name | Function | Key Components | Protons Pumped | ATP Contribution |
|---|---|---|---|---|---|
| I | NADH Dehydrogenase | Oxidizes NADH, transfers electrons to ubiquinone, initiates proton pumping | FMN, iron-sulfur clusters, multiple subunits | 4 | Approximately 2.5 ATP per NADH |
| II | Succinate Dehydrogenase | Oxidizes FADH2 from succinate, transfers electrons to ubiquinone without pumping protons | FAD, iron-sulfur clusters, links to citric acid cycle | 0 | Approximately 1.5 ATP per FADH2 |
| III | Cytochrome bc1 Complex | Transfers electrons from ubiquinol to cytochrome c via Q cycle, pumps protons | Cytochromes b and c1, iron-sulfur protein | 4 | Contributes to overall gradient for ATP synthesis |
| IV | Cytochrome c Oxidase | Final transfer of electrons from cytochrome c to oxygen, forming water and pumping protons | Heme groups, copper ions | 4 | Essential for completing the chain and preventing backup |
| V | ATP Synthase | Uses proton gradient to synthesize ATP from ADP and Pi through rotational catalysis | F0 (proton channel) and F1 (catalytic head) subunits | Uses protons (no pumping) | Produces 1 ATP per ~3-4 protons |
This table outlines the five major complexes, drawing from detailed biochemical studies that emphasize their sequential roles in electron transfer and energy harvesting. Each complex’s structure, involving metal centers and cofactors, ensures efficient redox reactions while minimizing harmful radical formation.
FAQ 3: How does aerobic respiration compare to anaerobic respiration in terms of process and efficiency?
Aerobic respiration is a comprehensive process that fully oxidizes glucose using oxygen, occurring in three main stages: glycolysis, the citric acid cycle, and oxidative phosphorylation. It takes place in the cytoplasm and mitochondria, yielding a high amount of ATP through efficient electron transfer. In contrast, anaerobic respiration bypasses oxygen, relying on alternative electron acceptors like nitrate or sulfate in some microbes, or fermenting to regenerate NAD+ in others, limiting it mostly to glycolysis with lower energy output.
- Energy Yield: Aerobic processes can produce up to 38 ATP per glucose molecule, making it far more efficient for sustained energy needs in complex organisms, while anaerobic yields only 2-4 ATP, suitable for quick bursts or oxygen-scarce environments.
- Byproducts: Aerobic respiration produces carbon dioxide and water, harmless wastes, whereas anaerobic can yield lactate (causing muscle fatigue) or ethanol and CO2 in yeast.
- Evolutionary Aspects: Aerobic respiration evolved after the Great Oxidation Event, allowing for larger, multicellular life, while anaerobic predates it, supporting early microbes in anoxic conditions.
- Cellular Locations: Both start in the cytoplasm, but aerobic extends to mitochondria for oxidative steps, enhancing efficiency through compartmentalization.
This comparison underscores why aerobic respiration dominates in oxygen-rich settings, supporting advanced life forms, though anaerobic pathways remain crucial for adaptability in low-oxygen scenarios.
FAQ 4: Why is oxygen essential for oxidative phosphorylation and what happens without it?
Oxygen plays a pivotal role in oxidative phosphorylation as the terminal electron acceptor in the electron transport chain, ensuring the continuous flow of electrons and preventing chain congestion. Without oxygen, electrons would accumulate, halting the process and forcing cells to rely on inefficient alternatives. Its high electronegativity allows it to efficiently pull electrons through the complexes, releasing energy for proton pumping and ATP synthesis. In the final step at complex IV, four electrons combine with oxygen and protons to form water: $$4\text{e}^- + \text{O}_2 + 4\text{H}^+ \rightarrow$$ $$ 2\text{H}_2\text{O}$$, a reaction that maintains the chain’s momentum.
In oxygen-deprived conditions, such as during intense exercise or in hypoxic tissues, cells switch to anaerobic metabolism, producing lactate and minimal ATP, leading to fatigue and acidosis. Chronic hypoxia can trigger adaptive responses like increased mitochondrial biogenesis, but prolonged absence of oxygen risks cellular damage from backed-up electrons forming reactive oxygen species. Evolutionarily, oxygen’s integration into metabolism post the rise of atmospheric levels enabled a quantum leap in energy efficiency, supporting the diversification of aerobic life.
This dependency on oxygen highlights its dual nature: vital for energy yet a source of oxidative stress if not managed, as excess can generate harmful radicals, linking to aging and disease processes.
FAQ 5: What are the consequences of disruptions in oxidative phosphorylation?
Disruptions in oxidative phosphorylation can stem from genetic mutations, toxins, or aging, profoundly impacting cellular energy and overall health. When the electron transport chain falters, ATP production drops, forcing cells into metabolic stress and reliance on glycolysis, which is unsustainable for energy-intensive organs like the brain and heart.
- Energy Deficit: Reduced ATP leads to fatigue, muscle weakness, and impaired organ function, as seen in conditions where complexes like I or IV are defective.
- Oxidative Stress: Electron leaks generate reactive oxygen species, damaging DNA, proteins, and lipids, accelerating cellular aging and contributing to neurodegeneration.
- Metabolic Shifts: Cells may accumulate intermediates like lactate, causing acidosis, or trigger apoptosis if damage is severe.
- Disease Links: Associated with mitochondrial myopathies, neurological disorders, and even cancer, where altered oxidative phosphorylation supports tumor survival in hypoxic environments.
Addressing these disruptions often involves mitigating factors like inflammation or enhancing antioxidant defenses to restore balance.
FAQ 6: How has oxidative phosphorylation influenced the evolution of life on Earth?
The emergence of oxidative phosphorylation represents a cornerstone in biological evolution, transitioning life from simple anaerobic forms to complex aerobic organisms capable of harnessing vast energy reserves. Around 2.4 billion years ago, during the Great Oxidation Event, cyanobacteria’s photosynthetic activity flooded the atmosphere with oxygen, setting the stage for this metabolic innovation. Early prokaryotes adapted by incorporating oxygen into respiration, dramatically increasing ATP yields from 2 per glucose in anaerobic processes to up to 38 in aerobic ones, fueling greater complexity and size.
This endosymbiotic event, where an aerobic bacterium was engulfed by a host cell about 1.5 billion years ago, birthed mitochondria, embedding oxidative phosphorylation in eukaryotic lineages. It enabled the Cambrian explosion, diversifying multicellular life by supporting energy-demanding traits like mobility and nervous systems. In contrast, anaerobic microbes persisted in niches like deep-sea vents, using alternative acceptors, but lacked the efficiency for advanced forms.
Today, this legacy persists in our cells, where mitochondrial DNA echoes bacterial origins, and disruptions mimic evolutionary bottlenecks, linking to modern diseases. Oxidative phosphorylation’s evolution underscores oxygen’s transformative power, shaping biodiversity from microbes to mammals.
FAQ 7: What mitochondrial diseases are associated with defects in oxidative phosphorylation?
| Disease | Primary Defect | Genetic Cause | Main Symptoms | Treatment Approaches | Prevalence |
|---|---|---|---|---|---|
| MELAS (Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like Episodes) | Complex I or IV | mtDNA mutations (e.g., MT-ND1) | Stroke-like episodes, seizures, muscle weakness, lactic acidosis | Symptomatic care, antioxidants like CoQ10 | 1 in 4,000 |
| MERRF (Myoclonic Epilepsy with Ragged Red Fibers) | Multiple complexes | mtDNA tRNA mutations | Myoclonus, epilepsy, ataxia, ragged red muscle fibers | Anticonvulsants, supportive therapy | Rare, <1 in 100,000 |
| Leigh Syndrome | Complexes I, IV, or ATP synthase | Nuclear or mtDNA mutations | Developmental delay, brain lesions, respiratory failure | Nutritional support, thiamine | 1 in 40,000 |
| Kearns-Sayre Syndrome | Multiple complexes | Large mtDNA deletions | Ophthalmoplegia, heart block, pigmentary retinopathy | Pacemakers, hormone replacement | Rare |
| NARP (Neuropathy, Ataxia, Retinitis Pigmentosa) | ATP synthase (Complex V) | mtDNA mutations | Neuropathy, ataxia, vision loss | Symptomatic, physical therapy | Very rare |
These diseases highlight the critical role of oxidative phosphorylation, with defects often leading to multisystem failures due to energy shortages. Early diagnosis via genetic testing can guide management.
FAQ 8: What natural methods can enhance mitochondrial function and oxidative phosphorylation?
Boosting mitochondrial health naturally involves lifestyle and dietary choices that reduce oxidative stress, support energy production, and promote biogenesis. Regular physical activity, particularly aerobic exercise, stimulates mitochondrial adaptations, increasing their number and efficiency in muscles.
- Nutrient-Rich Diet: Consume foods high in antioxidants like berries, leafy greens, and nuts to combat ROS; include CoQ10 from organ meats or supplements for electron transport support.
- Intermittent Fasting or Calorie Restriction: These practices trigger mitophagy, clearing damaged mitochondria and enhancing oxidative phosphorylation.
- Adequate Sleep and Stress Management: Quality rest regulates circadian rhythms affecting mitochondrial dynamics; techniques like meditation lower cortisol, preserving function.
- Supplements: Consider resveratrol from red grapes or PQQ for biogenesis; magnesium from seeds aids ATP synthesis.
- Cold Exposure: Brief cold showers activate brown fat, upregulating mitochondrial activity for thermogenesis.
Integrating these habits can improve energy levels and resilience against age-related decline.
FAQ 9: What is the ATP yield comparison between aerobic and anaerobic respiration?
| Aspect | Aerobic Respiration | Anaerobic Respiration |
|---|---|---|
| ATP per Glucose | 30-38 (including glycolysis, citric cycle, and oxidative phosphorylation) | 2-4 (mainly from glycolysis; fermentation regenerates NAD+) |
| Stages Involved | Glycolysis, Citric Acid Cycle, Electron Transport Chain | Glycolysis followed by fermentation (lactate or ethanol) |
| Efficiency | High; fully oxidizes glucose to CO2 and H2O | Low; partial oxidation, leaving energy in byproducts |
| Oxygen Requirement | Required as final electron acceptor | None; uses alternative acceptors or none in fermentation |
| Evolutionary Context | Evolved post-oxygen rise; supports complex life | Ancient; suits oxygen-poor environments, quick energy bursts |
| Examples | Muscle cells during endurance; most eukaryotes | Yeast in brewing; muscles in sprints |
Aerobic respiration’s superior yield enabled evolutionary advancements, while anaerobic provides rapid, albeit limited, energy.
FAQ 10: What are the recent breakthroughs in oxidative phosphorylation research as of 2025?
As of 2025, research into oxidative phosphorylation has unveiled innovative insights, particularly in its links to disease and therapeutic potential. Studies have highlighted how defects in this process drive hypermetabolism in mitochondrial diseases, accelerating aging through telomere shortening and epigenetic changes, prompting trials of metabolic modulators to extend lifespan. In cancer, emerging therapies target upregulated oxidative phosphorylation in resistant tumors, with inhibitors like IACS-010759 showing promise in starving cells by blocking complex I.
Advancements in imaging and proteomics, such as mass spectrometry for metabolite quantification and blue native PAGE for complex assembly, have refined diagnostics, enabling precise OXPHOS assessments. Rhodoquinone’s discovery in mammalian mitochondria offers flexibility under hypoxia, inspiring ischemia treatments. Immune connections reveal OXPHOS remodeling in T-cells, enhancing vaccine efficacy.
These developments, from 2024-2025, emphasize oxidative phosphorylation’s role in immunity, neurodegeneration, and bioenergetics, paving the way for personalized interventions like CRISPR-edited mitochondrial genes to correct defects.
FAQ 11: What are the latest breakthroughs in oxidative phosphorylation research as of 2025?
Recent research into oxidative phosphorylation has brought forth groundbreaking insights that challenge long-held beliefs about mitochondrial function and energy production. For instance, studies have revealed that this process can occur efficiently even in uncoupled mitochondria, particularly in brown adipose tissue, where new techniques have shown how heat generation and ATP synthesis coexist without strict dependency on membrane potential. This finding overturns traditional models, suggesting that mitochondrial bioenergetics in thermogenic tissues like brown fat operate with greater flexibility, potentially opening doors to treatments for metabolic disorders such as obesity.
Another significant advance involves the role of regulatory proteins like Foxp3 in adapting immune cells to harsh environments by boosting oxidative phosphorylation and NAD oxidation. In regulatory T cells, this enhancement allows survival in low-glucose, high-lactate conditions, linking energy metabolism directly to immune function. Such discoveries are pivotal for understanding chronic inflammation and autoimmune diseases, where mitochondrial efficiency could be a key therapeutic lever.
Furthermore, investigations into structural diversity and evolutionary constraints of oxidative phosphorylation proteins have mapped out how these complexes vary across species while maintaining core functions. By analyzing protein residues at evolutionary and population levels, researchers have identified conserved sites that resist mutations, providing clues for designing drugs that target dysfunctional variants in human diseases.
In cancer research, oxidative phosphorylation emerges as a vulnerability in endocrine therapy-resistant breast cancers, where persister cells rely on it for survival amid energy depletion. Metabolic tracing shows that inhibiting this pathway could eliminate resistant cells, highlighting a shift toward mitochondria-focused anticancer strategies.
Finally, explorations in osteoarthritis have demonstrated that shifting metabolism toward oxidative phosphorylation via PDK2 inhibition reduces cartilage degradation, offering a novel approach to joint health. These 2025 advancements collectively underscore oxidative phosphorylation’s dynamic role in health, disease, and evolution, paving the way for personalized medicine.
FAQ 12: How does exercise influence oxidative phosphorylation in the body?
Exercise profoundly impacts oxidative phosphorylation by enhancing mitochondrial efficiency and capacity, adapting the body to meet increased energy demands. During physical activity, muscles require more ATP, prompting the electron transport chain to ramp up electron flow and proton pumping, which in turn boosts ATP synthesis. Regular training, especially endurance exercises, leads to mitochondrial adaptations that improve overall energy production.
- Intensity Matters: High-intensity interval training increases oxidative phosphorylation efficiency in a temperature-dependent way, with studies showing better adaptations in cooler environments due to enhanced enzyme activity in skeletal muscle.
- Biogenesis Boost: Endurance workouts promote mitochondrial fission and mitophagy, clearing damaged organelles while increasing oxidative phosphorylation proteins, independent of age.
- Metabolic Shifts: Exercise optimizes ATP production by improving electron transport chain coupling, raising efficiency and reducing waste, which helps in performance enhancement.
- Potential Downsides: Excessive high-volume exercise can impair mitochondrial function, leading to glucose intolerance if not balanced with recovery.
- Long-Term Benefits: Consistent activity increases mitochondrial volume by 40-50%, paralleling improved respiration and reducing fatigue in daily life.
These changes not only elevate physical performance but also contribute to better metabolic health, lowering risks for conditions like diabetes.
FAQ 13: What mechanisms link oxidative phosphorylation to the aging process?
| Mechanism | Description | Impact on Aging | Supporting Evidence |
|---|---|---|---|
| Reduced ATP Production | Aging diminishes oxidative phosphorylation efficiency, leading to lower energy output from mitochondria. | Contributes to cellular fatigue, tissue decline, and frailty. | Studies show substrate oxidation impairs with age, increasing toxic intermediates. |
| Increased ROS Generation | Electron leaks in the ETC produce more reactive oxygen species, damaging cellular components. | Accelerates DNA mutations, protein misfolding, and lipid peroxidation. | ROS levels rise, linking to lifespan determinants via the free radical theory. |
| Mitochondrial DNA Damage | Accumulated mutations in mtDNA disrupt ETC complexes. | Impairs heart and brain function, exacerbating age-related diseases. | Interfibrillar mitochondria in aging hearts show greater oxidative phosphorylation decline. |
| Antioxidant Defense Decline | Lower enzymatic activity fails to neutralize ROS effectively. | Heightens oxidative stress, promoting senescence and inflammation. | ATP drop and ROS rise coincide with weakened defenses in aging cells. |
| Flux Control Changes | Aging alters control coefficients in the oxidative phosphorylation pathway. | Reduces adaptability to energy demands, affecting organ healthspan. | Investigations reveal pathway control shifts, impacting cellular energy. |
This table illustrates how oxidative phosphorylation’s dysfunction drives aging hallmarks, emphasizing mitochondria’s central role in longevity.
FAQ 14: What are promising therapeutic targets in electron transport chain disorders?
Therapeutic targeting of the electron transport chain holds immense potential for treating disorders rooted in mitochondrial dysfunction, such as cardiovascular diseases and neurodegeneration. One key area focuses on complex I, where dysregulation contributes to heart failure and ischemia; inhibitors or modulators like those affecting CI could restore balance and prevent tissue damage. Strategies include bypassing faulty complexes with alternative electron carriers, enhancing ATP production while minimizing ROS.
Another approach involves antioxidants and neuroprotective agents that directly address ETC impairments, offering broad applications in neurodegenerative conditions. For instance, coenzyme Q10 and its analogs improve electron transfer, supporting therapies for mitochondrial myopathies by bolstering chain function.
In cancer, ETC components like complex I serve as vulnerabilities; drugs repurposed to inhibit oxidative phosphorylation in tumor cells, such as in resistant breast cancers, show efficacy in clinical trials. This selective targeting exploits cancer’s metabolic reliance, sparing healthy cells.
Emerging therapies also explore immune-specific mitochondrial interventions, where modulating ETC in immune cells could alleviate inflammation-related diseases. Overall, these targets promise personalized treatments, combining gene therapy with small molecules to correct ETC defects.
FAQ 15: How does oxidative phosphorylation compare to photosynthesis in energy processes?
Oxidative phosphorylation and photosynthesis are mirror-image processes in energy conversion, both relying on electron transport chains to create proton gradients for ATP synthesis. While oxidative phosphorylation occurs in mitochondria to break down nutrients and produce ATP using oxygen, photosynthesis in chloroplasts uses light to build sugars, releasing oxygen as a byproduct.
- Electron Flow Direction: In oxidative phosphorylation, electrons move from high-energy donors like NADH to oxygen, releasing energy; in photosynthesis, light excites electrons from water to NADP+, capturing energy.
- Energy Source: Oxidative phosphorylation draws from organic molecules, whereas photosynthesis harnesses sunlight.
- ATP Mechanism: Both use chemiosmosis, but photophosphorylation is light-driven, differing from the redox-driven oxidative process.
- Byproducts: Oxidative yields CO2 and water; photosynthesis produces O2 and glucose.
- Evolutionary Link: Both evolved from ancient bacterial systems, sharing structural similarities in complexes.
This comparison highlights their complementary roles in the global energy cycle.
FAQ 16: What antioxidants help combat reactive oxygen species from the electron transport chain?
| Antioxidant | Source | Mechanism Against ROS | Benefits in ETC-Related Issues |
|---|---|---|---|
| Superoxide Dismutase (SOD) | Endogenous enzyme, supplements | Converts superoxide to hydrogen peroxide and oxygen. | Reduces ETC leaks, protecting against oxidative stress in aging. |
| Catalase | Liver, supplements | Breaks down hydrogen peroxide into water and oxygen. | Prevents peroxide buildup from complex I/III, aiding cellular health. |
| Glutathione Peroxidase | Selenium-rich foods | Reduces peroxides using glutathione. | Maintains redox balance, mitigating ETC-derived damage. |
| Vitamin E (Tocopherol) | Nuts, oils | Scavenges lipid peroxyl radicals in membranes. | Shields mitochondrial lipids from ROS, preserving ETC integrity. |
| Vitamin C (Ascorbate) | Fruits, vegetables | Neutralizes various ROS, regenerates other antioxidants. | Supports ETC function by reducing oxidative toxicity. |
This table outlines key antioxidants, emphasizing their role in neutralizing ETC-generated ROS for better health.
FAQ 17: What factors regulate mitochondrial biogenesis in relation to oxidative phosphorylation?
Mitochondrial biogenesis, the process of creating new mitochondria, is tightly regulated to support oxidative phosphorylation demands, especially in energy-hungry tissues. Key regulators include PGC-1α, a master transcription factor that coordinates nuclear and mitochondrial gene expression, enhancing ETC components for ATP production. AMPK, acting as an energy sensor, activates biogenesis during low-energy states like exercise, ensuring oxidative capacity matches needs.
Other factors involve hormonal inputs, such as thyroid hormones and PPARγ, which integrate signals for adaptive thermogenesis and uncoupling proteins. Retrograde signaling from mitochondria, via ROS or calcium, fine-tunes nuclear responses, maintaining balance.
Aging reduces AMPK activity, impairing biogenesis and thus oxidative phosphorylation, linking to declined healthspan. Tom70 proteins also regulate by facilitating protein import, influencing biogenesis under stress.
These mechanisms highlight biogenesis as a dynamic process essential for metabolic adaptation.
FAQ 18: Which drugs impact oxidative phosphorylation and how?
Certain drugs interact with oxidative phosphorylation, either therapeutically or as side effects, affecting mitochondrial energy production. For example, non-steroidal anti-inflammatory drugs like diclofenac can uncouple the process, disrupting proton gradients and potentially leading to toxicity.
- Anticancer Agents: Compounds targeting ETC, like those inhibiting complex I, exploit cancer’s reliance on oxidative phosphorylation for selective killing.
- Antidiabetics: Metformin mildly inhibits complex I, shifting metabolism and aiding diabetes management.
- Antiarrhythmics: Amiodarone induces phospholipidosis, impairing ETC and causing liver issues.
- Toxins as Models: Cyanide blocks complex IV, halting ATP, illustrating severe inhibition effects.
- Repurposed Drugs: Thalidomide derivatives may modulate for neuroprotection, balancing benefits and risks.
Understanding these interactions is crucial for safe prescribing.
FAQ 19: How evolutionarily conserved is oxidative phosphorylation across species?
| Aspect | Conservation Level | Examples | Implications |
|---|---|---|---|
| Core Complexes | Highly conserved | Similar ETC structures in bacteria to humans. | Reflects early evolutionary origin from endosymbiosis. |
| Protein Residues | Variable with constraints | OXPHOS residues show structural limits at population levels. | Aids in predicting disease mutations. |
| Gene Dynamics | Nuclear-mitochondrial interplay | Faster evolution in Hymenoptera OXPHOS genes. | Influences adaptation in diverse clades. |
| Environmental Response | Conserved physiology | Mitochondrial function adapts to variation in conservation efforts. | Guides species preservation strategies. |
| Convergent Evolution | In specialized clades | Similar OXPHOS changes in electric fish. | Supports unique traits like bioelectrogenesis. |
This table shows oxidative phosphorylation’s deep conservation, essential for life across kingdoms.
FAQ 20: What future directions hold for mitochondrial oxidative phosphorylation research?
Future research in mitochondrial oxidative phosphorylation is poised to revolutionize treatments for age-related and metabolic diseases by targeting dynamics like fission and fusion to combat oxidative stress-induced damage. Advances in single-cell omics will dissect cell-specific dysfunctions, enabling precision medicine for conditions like heart failure.
Gene therapy innovations, including CRISPR for mtDNA edits, aim to correct defects directly, expanding from current cofactor therapies. Integrating epigenetics with mitochondrial function could uncover how lifestyle influences OXPHOS longevity.
In inflammation and immunity, exploring OXPHOS remodeling in T-cells promises enhanced therapies for autoimmune disorders. Finally, addressing dysfunction in aging through targeted interventions like mitochondrial transplants holds potential for extending healthspan.
Acknowledgement
The development of the article “Oxidative Phosphorylation: Electron Transport Chain, Energy Synthesis, and Health Significance” was made possible through the wealth of scientific knowledge available from numerous reputable sources. The Examsmeta.com website expresses its gratitude to Nature (www.nature.com), ScienceDirect (www.sciencedirect.com), PubMed (pubmed.ncbi.nlm.nih.gov), and MDPI (www.mdpi.com) for providing access to peer-reviewed studies and comprehensive reviews that enriched the content with cutting-edge insights into mitochondrial function, electron transport chain dynamics, and their implications for health and disease. Their rigorous publications ensured the accuracy and depth of the information presented, making this article a reliable resource for understanding this critical biological process.
Key Contributions from Sources:
- Nature: Provided in-depth research on evolutionary aspects of oxidative phosphorylation and recent breakthroughs in mitochondrial dynamics.
- ScienceDirect: Offered detailed studies on the biochemical mechanisms of the electron transport chain and its role in disease pathology.
- PubMed: Supplied clinical data and reviews linking oxidative phosphorylation dysfunction to mitochondrial disorders and aging.
- MDPI: Contributed open-access articles on therapeutic targets and antioxidants impacting mitochondrial energy production.

