Cell signaling is one of the most fascinating processes in biology, allowing tiny cells in our bodies to communicate and coordinate like a well-orchestrated team. Imagine a bustling city where residents send messages to each other to get things done—that’s essentially what happens inside us every second. Without this communication, our bodies couldn’t function properly, from digesting food to fighting off infections. Cells aren’t isolated islands; they rely on signals to share information, request help, or trigger actions in other cells. This process ensures that specialized cells, each with their unique jobs, can work together seamlessly.
In the world of cell signaling, chemicals called signaling molecules or ligands play the starring role. These molecules are released by one cell and travel to others, binding to specific receptors and sparking a chain of events inside the receiving cell. Depending on how far these signals travel and who they target, signaling is categorized into different types. Among them, paracrine signaling stands out as a local communicator, focusing on nearby cells rather than sending messages across the entire body. It’s efficient, quick, and vital for immediate responses in tissues.
Table of Contents
This article dives deep into paracrine signaling, exploring its definition, properties, mechanisms, and real-world examples. We’ll also look at how it differs from other signaling types, the characteristics of the ligands involved, and why it’s so important for health. Drawing from biological principles, we’ll include practical insights, comparisons, and even potential issues when this system goes awry. Whether you’re a student, a curious reader, or someone interested in how your body works, this comprehensive guide will break it down in simple terms.
What is Paracrine Signaling?
Paracrine signaling is a form of cell-to-cell communication where a cell releases signaling molecules that affect neighboring cells in the same tissue or local area. Unlike signals that zip through the bloodstream to distant parts of the body, paracrine signals stay close to home, diffusing through the extracellular fluid to reach nearby targets. This makes it perfect for quick, localized responses, such as coordinating tissue repair or inflammation.
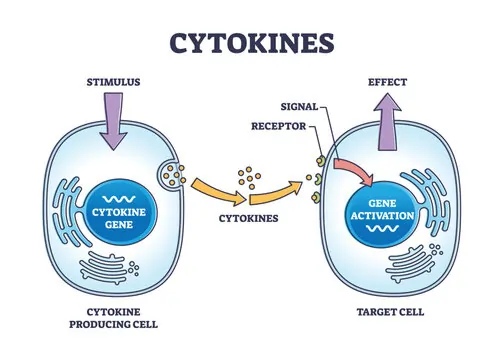
Think of it like neighbors chatting over a fence rather than shouting across town. The signaling cell secretes a chemical, often in response to a stimulus, and this chemical binds to receptors on adjacent cells, triggering changes in their behavior. These changes could include growth, division, or even death, depending on the context. Because the signals don’t travel far, they often have short lifespans, breaking down quickly to prevent unwanted effects elsewhere.
Paracrine signaling is widespread in the body, playing key roles in development, immune responses, and everyday maintenance. For instance, during wound healing, cells at the injury site release factors that tell surrounding cells to proliferate and rebuild tissue. This localized approach ensures efficiency and precision, avoiding the broader impacts that could come from system-wide signals.
Key Differences Between Paracrine, Autocrine, and Endocrine Signaling
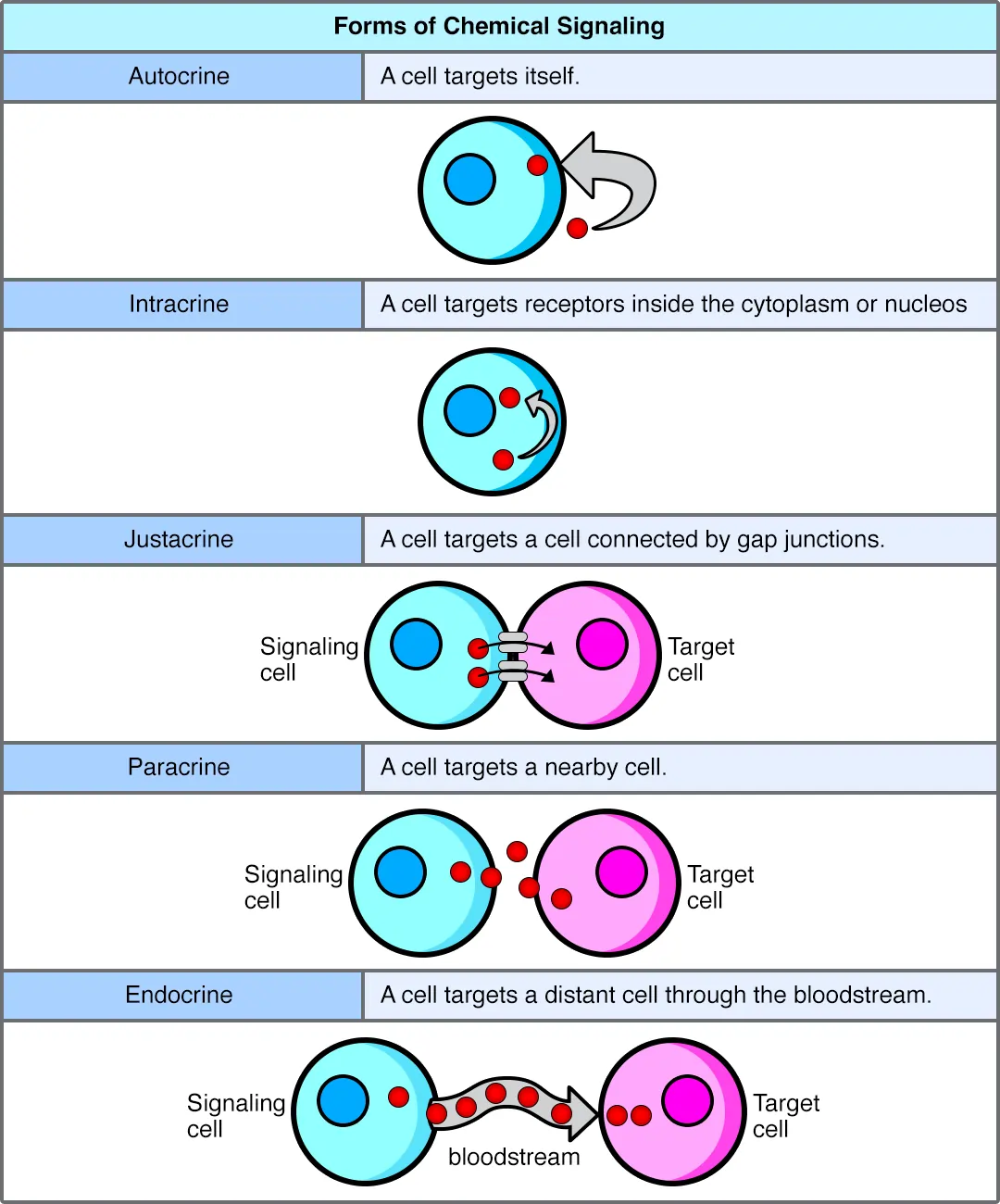
To fully appreciate paracrine signaling, it’s helpful to compare it with other major types: autocrine and endocrine signaling. Each has its own range, speed, and purpose, like different communication tools in a toolkit.
Autocrine signaling is when a cell signals itself, essentially talking to its own receptors with the molecules it releases. This self-stimulation is common in processes like cell growth or immune activation, where a cell reinforces its own actions.
Endocrine signaling, on the other hand, involves hormones released into the bloodstream, traveling long distances to affect far-off target cells. It’s slower but has a global reach, regulating things like metabolism or stress responses.
Paracrine signaling bridges the gap, acting locally but not on the sender cell itself. It’s faster than endocrine but more targeted than autocrine in group coordination.
Here’s a detailed comparison in table form to highlight the distinctions:
| Signaling Type | Distance Traveled | Target Cells | Speed of Action | Examples of Molecules Involved | Key Functions |
|---|---|---|---|---|---|
| Autocrine | Very short (same cell) | The signaling cell itself | Fast | Growth factors like EGF | Self-regulation, cell proliferation |
| Paracrine | Short (nearby cells) | Neighboring cells in local tissue | Fast | Neurotransmitters, cytokines | Local coordination, tissue repair, inflammation |
| Endocrine | Long (via bloodstream) | Distant cells throughout the body | Slower | Hormones like insulin | Systemic regulation, homeostasis |
This table shows how each type suits different biological needs. Understanding these differences helps explain why paracrine is ideal for rapid, site-specific tasks, while endocrine handles body-wide balance.
Properties of Paracrine Signaling
Paracrine signaling has several distinct properties that make it uniquely suited for local communication. These features ensure that signals are precise, temporary, and effective without causing widespread disruption.
- Localized Action: The affected cells must be in close proximity to the signaling cell, often just neighbors in the same tissue. This prevents the signal from spreading too far and causing unintended effects.
- Short Lifespan of Signals: The chemicals involved degrade quickly or are removed by nearby cells, limiting their duration and range. This rapid breakdown is crucial for fine-tuned control.
- Specificity Through Receptors: Only cells with the right receptors respond, adding a layer of selectivity. This means even in a crowded tissue, only the intended targets react.
- Diffusion-Based Travel: Signals move through the extracellular matrix via simple diffusion, not needing blood or nerves, which allows for swift transmission over short distances.
These properties highlight why paracrine signaling is so efficient in dynamic environments like developing embryos or inflamed tissues. Without them, cells might overcommunicate, leading to chaos.
In practical terms, these traits allow for quick adaptations. For example, in the immune system, paracrine signals from one white blood cell can rally nearby ones to attack a pathogen, containing the response to the infection site.
Characteristics of Ligands in Paracrine Signaling
Ligands are the messenger molecules in paracrine signaling, binding to receptors and kickstarting cellular responses. Their characteristics determine how effectively signals are sent and received.
Ligands in this context are often small proteins, peptides, or even gases, designed for short-range action. They can be ions or neutral molecules, forming complex structures around a central atom that helps in binding.
- Diverse Forms: Ligands aren’t limited to one type; they can be cations, anions, or neutral, allowing flexibility in signaling.
- Binding Affinity: They form bonds—covalent or ionic—with receptors, ensuring a secure attachment that triggers the signal cascade.
- Electron Dynamics: The central atom often accepts electron pairs, while other parts donate them, facilitating chemical interactions.
- Specificity: Each ligand matches particular receptors, like a key to a lock, preventing mix-ups.
These traits make ligands versatile tools in biology. For instance, some ligands are hydrophobic, diffusing easily through membranes, while others are hydrophilic, staying in the extracellular space.
Here’s a table outlining common characteristics of paracrine ligands:
| Characteristic | Description | Examples in Paracrine Ligands |
|---|---|---|
| Chemical Nature | Can be proteins, peptides, lipids, or gases | Cytokines (proteins), Nitric oxide (gas) |
| Size | Typically small to medium, aiding diffusion | Small peptides like bradykinin |
| Stability | Short half-life to limit range | Quickly degraded enzymes |
| Binding Type | Reversible or irreversible bonds with receptors | Ionic bonds in some ions |
| Origin | Synthesized and released by signaling cells | From immune or endothelial cells |
This variety ensures paracrine signaling can adapt to different tissues and needs.
Mechanism of Paracrine Signaling
The mechanism of paracrine signaling is a step-by-step process that’s both elegant and efficient. It starts when a stimulus prompts a cell to synthesize and secrete a ligand into the extracellular space.
Once released, the ligand diffuses to nearby cells, where it binds to specific receptors on the surface. This binding activates the receptor, often causing a conformational change that initiates an intracellular signaling cascade— a series of reactions amplifying the signal.
For example, the cascade might involve second messengers like cyclic AMP or calcium ions, leading to gene expression changes, enzyme activation, or cytoskeletal rearrangements. The response depends on the ligand and cell type.
To terminate the signal, ligands are degraded by enzymes or internalized, preventing prolonged activation. This mechanism’s speed and locality make it ideal for urgent tasks, like synaptic transmission in nerves.
In more detail, consider how ATP binding to P2Y receptors can trigger epidermal growth factor release, showcasing how paracrine loops enhance fidelity in wound healing or immune responses.
Examples of Paracrine Signaling in the Human Body
Paracrine signaling shines through in numerous bodily processes. Let’s explore some key examples, each illustrating its local power.
Enzyme Secretion
One classic example is enzyme release from glands, like salivary amylase from the salivary glands. This enzyme breaks down starches in the mouth, acting on nearby food particles before being inactivated further down the digestive tract. The short-range action ensures it works precisely where needed, degrading complex carbs into simpler sugars for absorption.
Other digestive enzymes, such as those from the pancreas, also use paracrine-like local effects to aid digestion in the small intestine.
Neurotransmitters
Neurotransmitters exemplify paracrine signaling at synapses. Acetylcholine, released from neuron endings, binds to receptors on adjacent muscle cells, causing contraction. This happens in skeletal muscles, where the signal crosses a tiny gap, ensuring rapid movement without affecting distant tissues.
Similarly, in the brain, neurotransmitters like GABA or glutamate coordinate local neural circuits, fine-tuning thoughts and reflexes.
Growth Factors
Growth factors are potent paracrine signals driving cell proliferation. For instance, epidermal growth factor (EGF) from one cell stimulates division in neighbors, crucial during embryonic development or skin repair.
Fibroblast growth factors (FGFs) play roles in angiogenesis, helping form new blood vessels locally to supply healing wounds.
Clotting Factors
In blood clotting, damaged vessels release factors like thromboxane A2, which acts on nearby platelets to aggregate and form a clot. This paracrine action stops bleeding quickly at the injury site without clotting the entire bloodstream.
Nitric Oxide
Nitric oxide (NO), a simple gas, is a paracrine messenger in blood vessels. Released by endothelial cells, it relaxes smooth muscle cells nearby, dilating vessels to improve blood flow. It’s vital for regulating blood pressure and responding to inflammation.
Cytokines and Immune Responses
Cytokines, such as interleukins from immune cells, signal nearby cells to mount defenses. For example, tumor necrosis factor (TNF) from macrophages alerts adjacent cells to inflammation, coordinating a local immune attack.
- Histamine in Allergies: Released by mast cells, it causes local swelling and redness by acting on nearby blood vessels.
- Prostaglandins: These lipid-derived signals mediate pain and fever locally in inflamed tissues.
These examples show how paracrine signaling underpins everything from daily functions to emergency responses.
Here’s an extensive table of paracrine signaling examples:
| Ligand/Molecule | Source Cell | Target Cell | Function | Body System Involved |
|---|---|---|---|---|
| Acetylcholine | Motor neurons | Skeletal muscle cells | Muscle contraction | Nervous/Muscular |
| Salivary Amylase | Salivary gland cells | Food particles in mouth | Carbohydrate breakdown | Digestive |
| EGF | Various epithelial cells | Neighboring epithelial cells | Cell growth and repair | Integumentary |
| Thromboxane A2 | Damaged endothelial cells | Platelets | Blood clot formation | Circulatory |
| Nitric Oxide | Endothelial cells | Smooth muscle cells | Vasodilation | Cardiovascular |
| Interleukins | Immune cells (e.g., T cells) | Nearby immune cells | Immune coordination | Immune |
| FGF | Fibroblasts | Endothelial cells | Angiogenesis | Developmental |
| Histamine | Mast cells | Blood vessels | Inflammation and allergy response | Immune |
| Prostaglandins | Various cells in inflamed tissue | Sensory neurons | Pain and fever mediation | Inflammatory |
| TNF | Macrophages | Adjacent cells in tissue | Apoptosis and inflammation | Immune |
This table captures the diversity, emphasizing paracrine’s role across systems.
Importance of Paracrine Signaling
Paracrine signaling is indispensable for maintaining bodily harmony. It enables cells to communicate locally, ensuring tissues respond appropriately to changes without involving the whole organism.
Without it, processes like wound healing would falter—cells couldn’t coordinate repair efficiently. In the immune system, it allows precise attacks on invaders, preventing overreactions that could harm healthy tissue.
It’s also key in development, where signals guide cell differentiation and organ formation. In adults, it supports ongoing maintenance, like bone remodeling or gut function.
Moreover, paracrine signals contribute to homeostasis, adjusting local environments for optimal performance. Disruptions can lead to issues, but its presence keeps things running smoothly.
Disorders Related to Paracrine Signaling
When paracrine signaling malfunctions, it can contribute to various disorders. Dysregulated signals often play roles in chronic conditions.
For example, in cancer, abnormal paracrine growth factors from tumor cells stimulate uncontrolled proliferation in neighbors, aiding tumor growth and metastasis.
In neurodegenerative diseases like Parkinson’s, impaired paracrine factors lead to neuron loss, as protective signals fail to support nearby cells.
Autoimmune disorders may involve overactive cytokines, causing excessive inflammation in tissues. Aging also disrupts paracrine balance, contributing to tissue dysfunction.
Environmental factors, like toxins such as arsenic, can alter mitochondrial function, affecting paracrine signals in muscle regeneration.
Therapies targeting these pathways, like stem cell treatments using paracrine effects, show promise for conditions like heart disease.
Role in Development and Tissue Repair
During embryonic development, paracrine signaling orchestrates cell fate. Factors like Sonic hedgehog guide limb formation by influencing nearby cells.
In tissue repair, it mobilizes stem cells and promotes angiogenesis. For instance, after injury, paracrine signals from macrophages recruit fibroblasts to lay down new matrix.
This role extends to regenerative medicine, where understanding paracrine mechanisms could enhance treatments for scars or organ damage.
Conclusion
Paracrine signaling is a cornerstone of cellular life, enabling local dialogues that keep our bodies functioning. From enzyme actions in digestion to immune defenses and beyond, its impact is profound. By appreciating its mechanisms, examples, and importance, we gain insight into biology’s intricate web. As research advances, harnessing paracrine pathways could revolutionize treatments for diseases, underscoring its enduring significance.
Frequently Asked Questions
FAQ 1: What is Paracrine Signaling in Biology?
Paracrine signaling is a vital form of cell communication where one cell releases chemical messengers that influence nearby cells within the same tissue or local area. These messengers, often called ligands or signaling molecules, diffuse through the extracellular fluid to reach their targets, ensuring that the effects are confined to a small region rather than spreading throughout the entire body. This localized approach allows for precise coordination among cells, which is essential for processes like inflammation or wound healing. Unlike broader signaling methods, paracrine signals are short-lived, degrading quickly to prevent unnecessary activation elsewhere in the organism.
In biological terms, paracrine signaling relies on the specificity of receptors on target cells. Only cells equipped with the appropriate receptors will respond to the signal, adding a layer of control to the process. For instance, in the immune system, certain cells release cytokines that act paracrine-style to alert neighboring immune cells to an infection, ramping up a targeted defense without alerting the whole body. This mechanism underscores how cells aren’t isolated but part of a dynamic network, constantly exchanging information to maintain tissue health and respond to changes.
The concept extends beyond human biology; it’s observed in various organisms, including bacteria, where paracrine-like signaling helps coordinate group behaviors. In humans, it plays a role in everything from regulating blood flow to supporting embryonic development, highlighting its evolutionary importance in multicellular life.
FAQ 2: How Does Paracrine Signaling Differ from Autocrine and Endocrine Signaling?
Understanding the distinctions between paracrine, autocrine, and endocrine signaling is key to grasping how cells communicate at different scales. Paracrine signaling involves short-distance messaging to nearby cells, autocrine targets the signaling cell itself, and endocrine reaches distant cells via the bloodstream.
| Aspect | Paracrine Signaling | Autocrine Signaling | Endocrine Signaling |
|---|---|---|---|
| Distance Traveled | Short, local diffusion to neighboring cells | Very short, acts on the same cell | Long, through bloodstream to distant targets |
| Speed of Response | Fast, due to proximity | Immediate, self-directed | Slower, as it relies on circulation |
| Duration of Effect | Brief, signals degrade quickly | Can be sustained for self-regulation | Longer-lasting, for systemic effects |
| Key Molecules Involved | Cytokines, growth factors, nitric oxide | Often growth factors like EGF | Hormones such as insulin or estrogen |
| Primary Functions | Local coordination, tissue repair, immune responses | Cell growth, survival, immune activation | Body-wide regulation, homeostasis, metabolism |
| Examples in Body | Neurotransmitter release at synapses, enzyme secretion in digestion | Cancer cells stimulating their own proliferation | Thyroid hormones affecting overall energy levels |
| Receptor Specificity | Targets specific nearby cells with matching receptors | Self-receptors on the producing cell | Widespread receptors on various distant cells |
| Evolutionary Role | Essential for tissue-level organization in multicellular organisms | Supports individual cell autonomy | Crucial for integrating organ systems in complex animals |
These differences ensure that cellular communication is efficient and tailored to specific needs, preventing overlap or confusion in signals. For example, while paracrine might handle a quick inflammatory response, endocrine manages broader hormonal balance.
FAQ 3: What is the Mechanism of Paracrine Signaling Step by Step?
Paracrine signaling operates through a series of coordinated steps that allow cells to communicate effectively over short distances. It begins when a signaling cell detects a stimulus, such as injury or infection, prompting it to synthesize and release specific ligands into the extracellular space. These ligands then diffuse locally, seeking out receptors on adjacent cells.
Once a ligand binds to its receptor, it triggers a conformational change in the receptor, activating intracellular pathways. This often involves second messengers like cyclic AMP or calcium ions, which amplify the signal and lead to cellular responses such as gene expression or enzyme activation.
The process concludes with signal termination to avoid overstimulation—ligands are degraded by enzymes or internalized by cells. This mechanism’s efficiency lies in its locality and speed, making it ideal for rapid adjustments in tissues.
- Stimulus Detection: The signaling cell senses an environmental cue and initiates ligand production.
- Ligand Release: Molecules are secreted via exocytosis into the nearby fluid.
- Diffusion and Binding: Ligands travel short distances and attach to specific receptors on target cells.
- Signal Transduction: Receptor activation sparks a cascade, often involving kinases or ion channels.
- Cellular Response: Targets alter behavior, like proliferating or secreting substances.
- Signal Shutdown: Quick degradation ensures the response is temporary and precise.
This step-by-step process highlights paracrine signaling’s role in maintaining tissue harmony.
FAQ 4: What Are Common Examples of Paracrine Signaling in the Human Body?
Paracrine signaling manifests in various bodily systems, showcasing its versatility in local cell interactions. One prominent example is the release of nitric oxide by endothelial cells in blood vessels, which relaxes nearby smooth muscle cells to dilate vessels and improve blood flow, crucial for regulating blood pressure.
Another involves cytokines in the immune system, where macrophages release interleukins to activate adjacent immune cells during an infection, coordinating a swift local defense.
In digestion, salivary glands secrete amylase that acts on nearby food particles in the mouth, breaking down carbohydrates efficiently before further processing.
- Thromboxane A2 in Blood Clotting: Released from damaged vessels, it signals platelets to aggregate and form clots at the injury site.
- Epidermal Growth Factor (EGF): Stimulates proliferation in neighboring epithelial cells for skin repair.
- Fibroblast Growth Factors (FGFs): Promote angiogenesis by encouraging new blood vessel formation in healing tissues.
- Histamine in Allergies: Mast cells release it to cause local inflammation and swelling in response to allergens.
- Prostaglandins: Mediate pain and fever in inflamed areas by acting on sensory neurons.
- Sonic Hedgehog Protein: Guides tissue patterning during development, like in tooth formation.
- Bone Morphogenetic Proteins (BMPs): Regulate bone growth and repair by influencing nearby osteoblasts.
These examples illustrate how paracrine signaling supports everyday functions and emergency responses.
FAQ 5: Why is Paracrine Signaling Important for Human Health?
Paracrine signaling is fundamental to human health because it enables precise, localized communication between cells, ensuring tissues respond appropriately to internal and external changes. Without it, coordinated actions like immune responses or tissue maintenance would falter, leading to inefficiencies or breakdowns in bodily functions. For instance, in the cardiovascular system, it helps regulate blood flow by allowing cells to signal neighbors for vessel adjustments, preventing issues like hypertension.
Moreover, paracrine signaling supports regenerative processes, such as healing wounds or repairing organs after injury. Stem cells often rely on paracrine factors to orchestrate recovery, releasing molecules that reduce inflammation and promote cell growth in the affected area. This not only speeds up healing but also minimizes scarring, contributing to overall tissue integrity.
In the context of disease prevention, paracrine signals play a defensive role by alerting cells to threats, like pathogens, and mobilizing resources without overtaxing the entire body. Disruptions in this signaling can lead to chronic conditions, but its proper function maintains homeostasis, underscoring its indispensable role in sustaining health throughout life.
FAQ 6: What Role Does Paracrine Signaling Play in Tissue Repair?
Paracrine signaling is a cornerstone in tissue repair, acting as the coordinator that rallies cells to fix damage efficiently. When an injury occurs, affected cells release signaling molecules like growth factors that diffuse to nearby cells, instructing them to proliferate, migrate, or differentiate as needed. This local messaging ensures the repair is focused, avoiding unnecessary activity in unaffected areas.
In processes like wound healing, paracrine signals from immune cells attract fibroblasts to produce new extracellular matrix, while others stimulate angiogenesis to restore blood supply. Over time, this leads to scar formation or regeneration, depending on the tissue type. Research shows that stem cell therapies leverage paracrine effects, where injected cells secrete factors to enhance native repair mechanisms without necessarily integrating into the tissue.
Overall, paracrine signaling’s role extends to minimizing inflammation and promoting resolution, making it essential for recovering from injuries ranging from minor cuts to major traumas.
FAQ 7: What Are the Characteristics of Ligands in Paracrine Signaling?
Ligands in paracrine signaling are diverse molecules tailored for short-range communication, each with properties that ensure effective local action.
| Characteristic | Description | Examples |
|---|---|---|
| Chemical Composition | Often proteins, peptides, lipids, or gases, allowing varied interactions | Cytokines (proteins), nitric oxide (gas) |
| Size and Solubility | Small to medium size for easy diffusion; can be hydrophobic or hydrophilic | Small peptides like bradykinin |
| Lifespan | Short half-life to restrict effects to local areas | Rapidly degraded growth factors |
| Binding Mechanism | Form reversible bonds with receptors, triggering cascades | Ionic or covalent interactions |
| Specificity | High affinity for particular receptors on target cells | FGF binding to tyrosine kinase receptors |
| Origin and Release | Synthesized in signaling cells and released via exocytosis | From immune or epithelial cells |
| Functional Diversity | Can stimulate growth, inflammation, or relaxation | BMPs for bone development |
| Regulation | Controlled by environmental stimuli like injury or pH changes | ATP release in response to stress |
These traits make ligands efficient messengers in paracrine contexts.
FAQ 8: Are Neurotransmitters Considered Paracrine Signals?
Yes, many neurotransmitters function as paracrine signals, particularly at synapses where they transmit information across short gaps between neurons or from neurons to muscles. For example, acetylcholine released from a motor neuron diffuses to nearby muscle cells, binding to receptors and causing contraction, embodying the local, rapid nature of paracrine action.
This dual role—neurotransmission and paracrine signaling—highlights how neurotransmitters aren’t just for nerves; they can influence surrounding cells in non-neural contexts too. In the pancreatic islets, neurotransmitters like glutamate act paracrine-style to regulate insulin secretion, showing their broader endocrine implications.
Overall, classifying neurotransmitters as paracrine signals emphasizes their importance in precise, localized communication, essential for reflexes, digestion, and even immune modulation.
FAQ 9: What Disorders Can Result from Dysregulated Paracrine Signaling?
Dysregulated paracrine signaling can contribute to numerous disorders by disrupting local cell harmony.
- Cancer: Overactive growth factors like FGF promote uncontrolled proliferation and tumor growth.
- Autoimmune Diseases: Excessive cytokines lead to chronic inflammation, as seen in rheumatoid arthritis.
- Neurodegenerative Conditions: Impaired signals in Parkinson’s cause neuron loss and motor issues.
- Fibrosis: Abnormal signaling expands scar tissue, affecting organs like the skin or lungs.
- Cardiovascular Problems: Faulty nitric oxide signaling contributes to hypertension or atherosclerosis.
- Metabolic Disorders: Disrupted signals in pancreatic islets impair insulin regulation, linking to diabetes.
- Aging-Related Issues: Declining paracrine efficiency leads to tissue dysfunction and frailty.
Addressing these often involves targeting specific pathways for therapy.
FAQ 10: How is Paracrine Signaling Involved in Embryonic Development?
Paracrine signaling is crucial during embryonic development, guiding cells to form complex structures through local cues. Early on, factors like Sonic hedgehog diffuse from organizing centers to pattern limbs and organs, ensuring proper differentiation and positioning.
As development progresses, paracrine signals coordinate tissue interactions, such as in tooth formation where FGF and BMP proteins work together to shape enamel and dentin. This signaling also regulates cell migration and apoptosis, sculpting the embryo without global disruptions.
In later stages, it supports organogenesis, like in the kidney where paracrine factors induce tubule formation. Overall, paracrine signaling’s precision fosters the intricate architecture of life from a single cell.
FAQ 11: How Does Paracrine Signaling Work in the Immune System?
Paracrine signaling plays a pivotal role in the immune system by enabling rapid, localized responses to threats like infections or injuries. When immune cells detect a pathogen, they release signaling molecules such as cytokines, which diffuse to nearby cells, alerting them to join the fight. For example, macrophages might secrete tumor necrosis factor-alpha, which acts on adjacent neutrophils to enhance their pathogen-killing abilities, creating a coordinated defense without involving the entire body. This localized communication ensures that inflammation is contained, preventing unnecessary damage to healthy tissues.
In more detail, paracrine signals in immunity often involve interleukins and interferons, which modulate the behavior of neighboring cells. During an inflammatory response, these molecules can prime immune cells for action, increasing their sensitivity to further signals or promoting migration to the infection site. Studies show that this signaling is crucial in fungal infections, where monocytes and neutrophils use paracrine cues to amplify their responses, demonstrating how interconnected immune networks rely on short-range messaging for efficiency.
Beyond acute responses, paracrine signaling contributes to chronic immune regulation, such as in autoimmune conditions where dysregulated cytokines lead to persistent inflammation. It also intersects with other systems, like in cancer, where immune cells’ paracrine factors can either suppress or promote tumor growth depending on the context. Overall, this mechanism underscores the immune system’s adaptability, allowing it to fine-tune reactions based on immediate environmental cues.
FAQ 12: What is the Difference Between Paracrine and Juxtacrine Signaling?
Paracrine and juxtacrine signaling are both forms of local cell communication, but they differ in how signals are transmitted and the proximity required between cells. Understanding these distinctions helps clarify how cells interact in tissues, from development to immune responses.
| Aspect | Paracrine Signaling | Juxtacrine Signaling |
|---|---|---|
| Mode of Transmission | Signals diffuse through extracellular fluid to nearby cells | Requires direct physical contact between cells via membrane-bound molecules |
| Distance Covered | Short distances, but not immediate contact; can span several cell lengths | Extremely close; cells must touch, often through gap junctions or ligands |
| Speed of Action | Relatively fast, but depends on diffusion rate | Instantaneous due to direct interaction |
| Examples of Molecules | Cytokines, growth factors, neurotransmitters | Notch ligands, integrins, cadherins |
| Key Functions | Coordinates local responses like inflammation or tissue repair | Essential for cell adhesion, embryonic patterning, and immune synapse formation |
| Receptor Interaction | Ligands bind to receptors after diffusion | Membrane-tethered ligands directly engage receptors on adjacent cells |
| Biological Contexts | Immune activation, wound healing, synaptic transmission | T-cell activation, neural development, stem cell niche maintenance |
| Evolutionary Role | Allows flexible communication in multicellular organisms | Promotes stable, contact-dependent organization in tissues |
These differences ensure that paracrine signaling offers broader local influence, while juxtacrine provides precise, contact-specific cues.
FAQ 13: Can Paracrine Signaling Be Targeted for Therapeutic Purposes?
Paracrine signaling holds immense promise for therapeutic interventions because it influences local cellular behaviors in diseases ranging from heart conditions to cancer. By modulating these short-range signals, treatments can focus on specific tissues without widespread side effects. For instance, stem cell therapies often harness paracrine factors released by injected cells to promote healing, such as reducing inflammation or stimulating regeneration in damaged organs.
Researchers are exploring ways to enhance or inhibit paracrine pathways for better outcomes. In cardiovascular disease, paracrine signals from endothelial cells can be targeted to improve heart remodeling post-injury.
- Cancer Therapies: Blocking paracrine hedgehog signaling in tumor stroma has shown potential to slow cancer progression by disrupting supportive environments.
- Stem Cell Applications: Adult stem cells’ paracrine effects, like secreting growth factors, are used in regenerative medicine to treat conditions like osteoarthritis.
- Nanoparticle Delivery: Biodegradable nanoparticles can deliver paracrine stimulants to embryonic stem cells, aiding tissue engineering.
- Autoimmune Modulation: Targeting cytokines in paracrine loops could reduce excessive immune responses in diseases like rheumatoid arthritis.
- Wound Healing: Enhancing paracrine factors from mesenchymal stem cells accelerates skin repair by promoting cell migration and angiogenesis.
- Neurological Disorders: Paracrine signals from transplanted cells might protect neurons in Parkinson’s, offering neuroprotective benefits.
These approaches highlight paracrine signaling’s versatility, with ongoing trials aiming to translate lab findings into clinical tools.
FAQ 14: How Does Paracrine Signaling Influence Cancer Development?
Paracrine signaling significantly impacts cancer development by facilitating communication between tumor cells and their surrounding microenvironment, often promoting growth and spread. Cancer cells release paracrine factors like growth hormones that recruit and activate nearby fibroblasts, turning them into cancer-associated fibroblasts which in turn secrete molecules supporting tumor progression. This bidirectional exchange creates a nurturing niche for the cancer, enhancing angiogenesis and immune evasion.
In breast cancer, for example, paracrine signals from stromal cells influence epithelial cell behavior, driving invasion and metastasis. Similarly, in pancreatic cancer, paracrine cues coordinate the metastatic cascade, from initial tumor formation to distant spread. Dysregulated signaling can lead to excessive cell proliferation, as seen when hedgehog pathways are hijacked, stimulating stromal cells to aid tumor survival.
Moreover, paracrine interactions involve immune modulation, where tumor-derived factors suppress local immune responses, allowing unchecked growth. Research indicates that targeting these signals, such as inhibiting platelet-derived growth factors, could disrupt fibroblast recruitment and slow malignancy. Understanding these mechanisms opens doors to therapies that interrupt the tumor’s supportive network, potentially improving outcomes in aggressive cancers.
FAQ 15: What Role Does Paracrine Signaling Play in the Nervous System?
Paracrine signaling is essential in the nervous system, facilitating local communication that supports everything from synaptic transmission to neuroprotection. It allows neurons and glial cells to coordinate responses without relying solely on electrical impulses.
| Aspect | Description | Examples in Nervous System |
|---|---|---|
| Primary Functions | Enables quick local adjustments in neural activity and repair | Neurotransmitter release at synapses for muscle contraction |
| Key Molecules Involved | Neurotransmitters, growth factors, cytokines | Acetylcholine, glutamate, nitric oxide |
| Cellular Interactions | Neurons signal to glia or nearby neurons via diffusion | Astrocytes releasing factors to modulate synaptic strength |
| Speed and Range | Fast over short distances, ideal for precise control | Synaptic cleft signaling in milliseconds |
| Developmental Role | Guides axon growth and synapse formation | BDNF promoting neuronal survival and differentiation |
| Pathological Impacts | Dysregulation linked to neurodegeneration | Reduced paracrine support in Alzheimer’s leading to neuron loss |
| Therapeutic Potential | Targets for drugs enhancing neuroregeneration | Stem cell paracrine factors for stroke recovery |
| Integration with Systems | Links nervous with immune and vascular systems | Nitric oxide from endothelium affecting neural blood flow |
This role emphasizes paracrine signaling’s contribution to neural plasticity and resilience.
FAQ 16: Are There Evolutionary Aspects to Paracrine Signaling?
Paracrine signaling has deep evolutionary roots, emerging as a key adaptation in multicellular organisms to enable coordinated cellular behaviors. In early life forms, simple diffusion of molecules allowed primitive cells to influence neighbors, fostering group survival strategies like quorum sensing in bacteria. As organisms grew more complex, this mechanism evolved to support tissue organization, where signals like growth factors ensured proper development and maintenance.
Over time, paracrine signaling diversified, integrating with other pathways to handle specialized functions. In mammals, it became crucial for responses like wound healing and immune coordination, reflecting evolutionary pressures for efficiency in larger bodies. Studies suggest that its conservation across species highlights its reliability, with similar molecules used in plants and animals for local communication.
Furthermore, evolutionary tweaks in paracrine networks may have driven speciation, as variations in signaling efficiency influenced traits like organ size or regenerative abilities. In cancer, evolutionary perspectives show how tumors exploit these ancient pathways for survival, underscoring paracrine signaling’s enduring impact on biological innovation.
FAQ 17: How Does Paracrine Signaling Affect Hormone Regulation?
Paracrine signaling intersects with hormone regulation by providing local fine-tuning that complements broader endocrine effects. In endocrine glands, paracrine factors from one cell type can modulate hormone secretion in adjacent cells, ensuring balanced outputs.
- Islet Cell Coordination: In the pancreas, paracrine signals between beta and alpha cells regulate insulin and glucagon release, maintaining blood sugar levels.
- Feedback Loops: Autocrine and paracrine cues create regulatory circuits, where secreted hormones influence nearby producers for precise control.
- GPCR Involvement: G-protein-coupled receptors mediate paracrine effects on hormone-secreting cells, adjusting outputs based on local needs.
- Immune-Endocrine Link: Cytokines act paracrine-style to alter hormone production during inflammation, linking stress responses.
- Reproductive Systems: In ovaries, paracrine factors like inhibins regulate follicle-stimulating hormone, aiding ovulation cycles.
- Thyroid Function: Local signals influence calcitonin release, impacting calcium homeostasis alongside parathyroid hormones.
- Adrenal Gland Dynamics: Paracrine interactions between cortical cells fine-tune cortisol production for stress adaptation.
This integration highlights paracrine signaling’s role in hormonal homeostasis.
FAQ 18: What Are Advanced Research Techniques for Studying Paracrine Signaling?
Advanced techniques have revolutionized the study of paracrine signaling, allowing precise observation of local cellular interactions. These methods provide insights into dynamic processes in real time.
| Technique | Description | Applications and Advantages |
|---|---|---|
| Proximal Culture | Cells are cultured in close proximity without direct contact to study secreted factors | Distinguishes paracrine from juxtacrine effects in cancer metastasis models |
| Microfluidics Devices | Reconfigurable chips simulate spatiotemporal dynamics of signaling | Tracks factor diffusion and responses in controlled environments |
| Live Imaging | Fluorescence-based tracking of secretion, diffusion, and receptor binding | Visualizes real-time paracrine events in tissues like pancreatic islets |
| Conditioned Medium Studies | Media from one cell type applied to another to assess paracrine influences | Identifies specific molecules in stem cell therapies |
| Single-Cell Sequencing | Analyzes gene expression to infer communication networks | Maps paracrine interactions in complex tissues like tumors |
| Fluorescence Correlation Spectroscopy | Measures diffusion rates of signaling molecules | Quantifies paracrine factor movement in extracellular spaces |
| Coculture Assays | Mixed cell cultures to observe bidirectional signaling | Studies immune-tumor paracrine crosstalk |
| Nanoparticle Delivery | Targeted release of stimulants for controlled paracrine activation | Enhances stem cell renewal studies |
These tools advance our understanding of paracrine mechanisms in health and disease.
FAQ 19: How Does Aging Impact Paracrine Signaling?
Aging profoundly affects paracrine signaling, leading to disruptions that contribute to tissue decline and disease susceptibility. As cells age, their ability to produce and respond to paracrine factors diminishes, often due to accumulated damage like oxidative stress. For instance, senescent cells release inflammatory cytokines via the senescence-associated secretory phenotype, which spreads senescence to neighbors through paracrine means, accelerating aging across tissues.
In the immune system, age-related changes in paracrine signals weaken responses, making older individuals more prone to infections. Similarly, in the musculoskeletal system, reduced paracrine support from stem cells impairs repair, leading to frailty. Research shows that restoring youthful paracrine environments, such as through co-culturing old cells with young ones, can rejuvenate function, highlighting potential anti-aging strategies.
Furthermore, organs like the thymus see paracrine dysregulation with age, shrinking and reducing immune output. This interconnected decline underscores how paracrine imbalances fuel a cycle of deterioration, but targeting these pathways could promote healthier longevity.
FAQ 20: What Future Directions Are There in Paracrine Signaling Research?
Future research in paracrine signaling is poised to uncover new therapeutic avenues by delving deeper into its mechanisms and applications. Scientists aim to map complex interorgan communications, using advanced models to understand how paracrine factors influence systemic health.
- Regenerative Medicine: Enhancing stem cell paracrine effects for better tissue repair in conditions like heart failure.
- Cancer Neuroscience: Exploring bidirectional paracrine signals between tumors and nerves to develop novel inhibitors.
- Live Imaging Advances: Improving techniques to track paracrine dynamics in vivo for real-time insights.
- Nanotechnology Integration: Using nanoparticles for targeted delivery of paracrine modulators in precision medicine.
- Organ Crosstalk Studies: Investigating cardiovascular and multi-organ paracrine networks for holistic disease treatments.
- Redox Signaling Focus: Examining paracrine redox mechanisms in aging and regeneration for antioxidant therapies.
- Single-Cell Technologies: Refining analyses to decode paracrine networks in heterogeneous tissues.
- Clinical Translations: Expanding trials on paracrine-based therapies for neurological and autoimmune disorders.
These directions promise to bridge basic science with clinical innovations, transforming how we approach disease.
Acknowledgement
The Examsmeta.com website expresses its sincere gratitude to the various reputable sources that provided invaluable insights and data for the article “Paracrine Signaling: Definition, Mechanism, and Key Examples.” Their comprehensive resources enriched the content, ensuring accuracy and depth in exploring the complexities of paracrine signaling. Special thanks go to the following:
- Nature (www.nature.com) for its detailed publications on cellular communication and signaling pathways, which offered a strong scientific foundation.
- ScienceDirect (www.sciencedirect.com) for providing access to peer-reviewed articles that enhanced the understanding of paracrine mechanisms and applications.
- PubMed (pubmed.ncbi.nlm.nih.gov) for its extensive database of biomedical research, crucial for referencing examples and therapeutic insights.
- Cell Press (www.cell.com) for its cutting-edge studies on molecular biology, particularly in cancer and developmental signaling.
- Journal of Clinical Investigation (www.jci.org) for its clinical perspectives on paracrine signaling in disease contexts.

