DNA, or deoxyribonucleic acid, stands as one of the most remarkable molecules in the living world. It serves as the blueprint for life, carrying the genetic instructions that guide the development, functioning, and reproduction of all organisms. From the tiniest bacteria to complex human beings, DNA’s unique structure and properties enable it to store vast amounts of information in a compact form. This molecule not only dictates traits like eye color and height but also orchestrates the production of proteins essential for cellular processes. Understanding DNA’s properties reveals how it maintains stability while allowing for the flexibility needed in biological functions.
In this comprehensive article, we’ll delve into its structural intricacies, physical behaviors, chemical attributes, and broader implications, drawing on established scientific insights to paint a complete picture.
Table of Contents
The discovery of DNA’s structure in the 1950s by scientists like James Watson and Francis Crick revolutionized biology. Their model of the double helix explained how genetic information could be replicated accurately during cell division. Today, we know DNA isn’t just a static entity; it interacts dynamically with its environment, responding to heat, chemicals, and proteins. These interactions underpin processes like gene expression and DNA replication, which are crucial for life. As we explore further, you’ll see how DNA’s properties make it both resilient and adaptable, ensuring the continuity of life across generations.
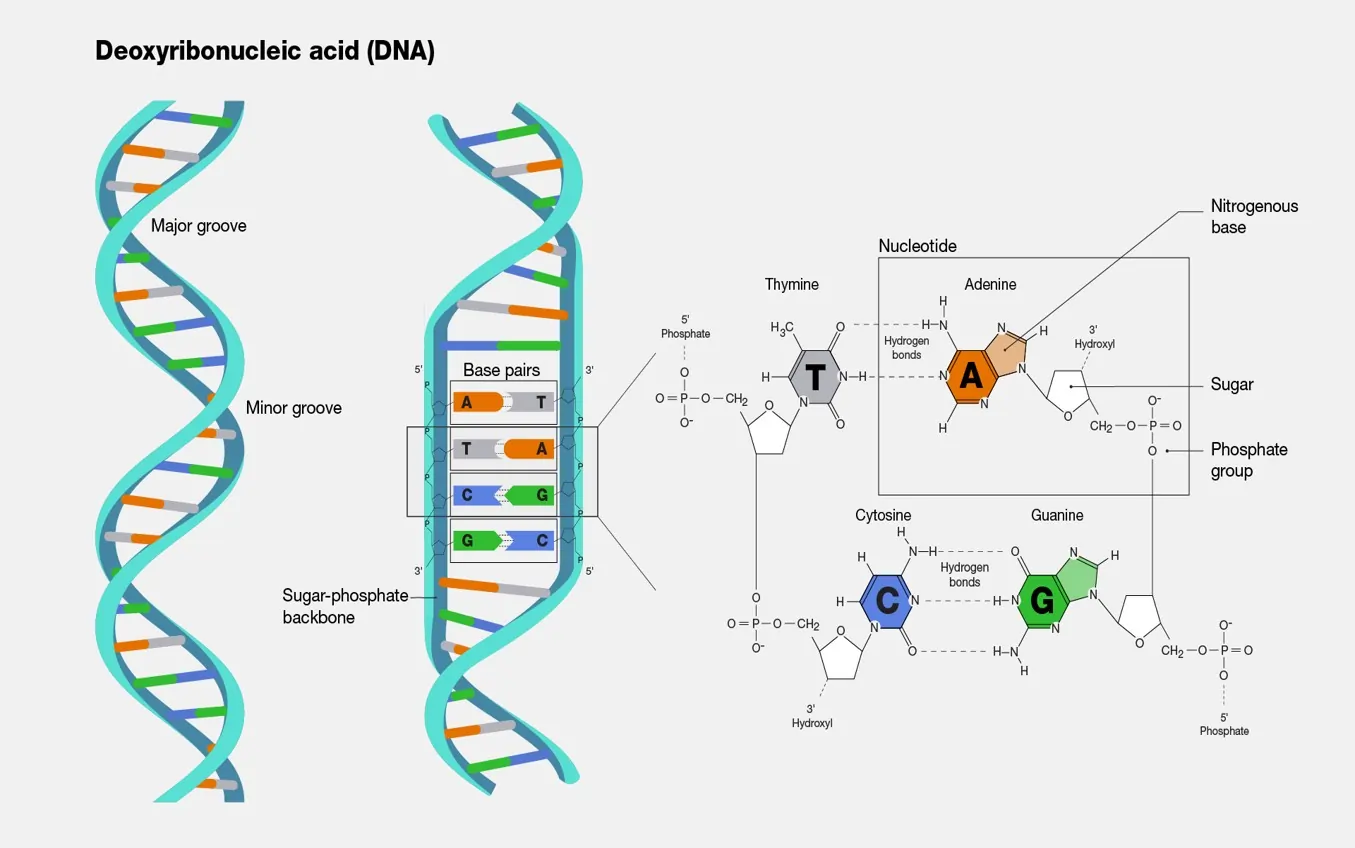
The Fundamental Structure of DNA
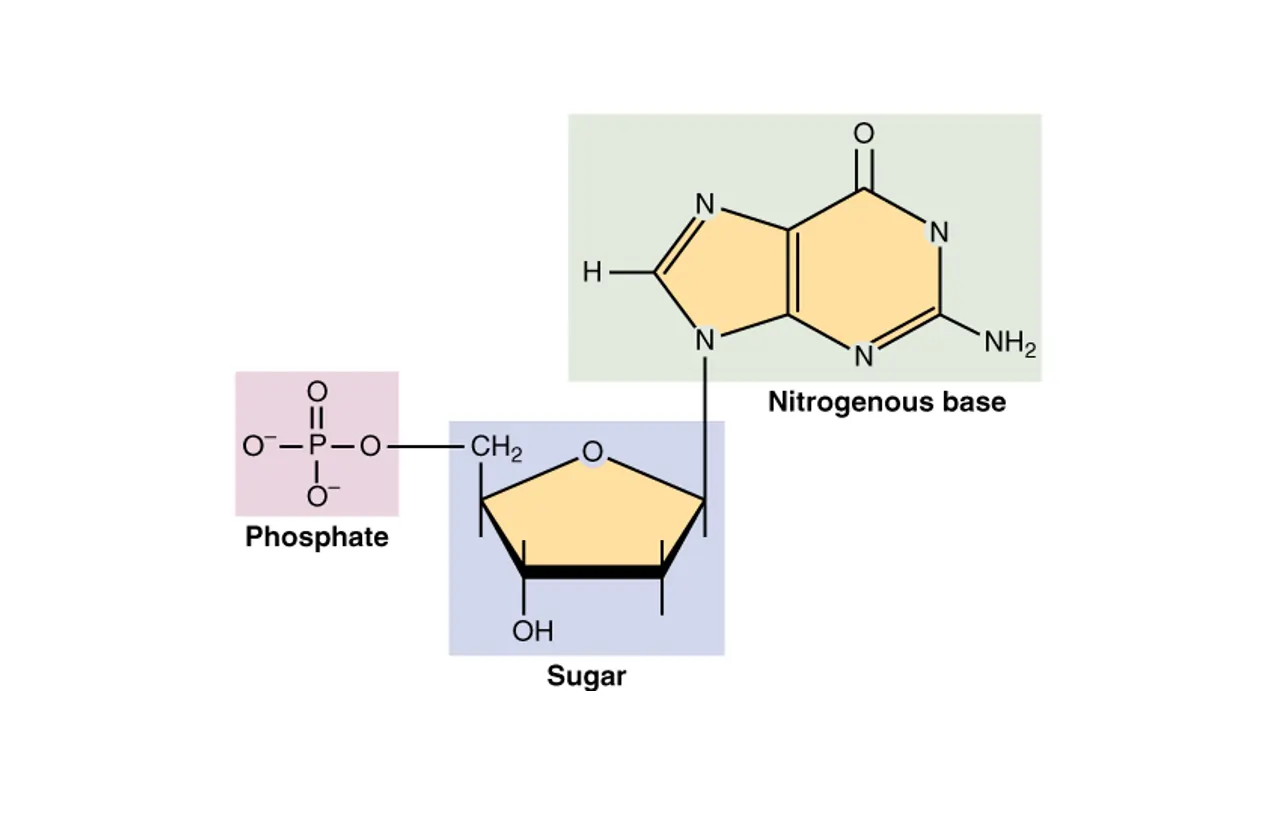
DNA is a long polymer made up of repeating units called nucleotides. Each nucleotide consists of three components: a five-carbon sugar called deoxyribose, a phosphate group, and one of four nitrogenous bases—adenine (A), thymine (T), cytosine (C), or guanine (G). These nucleotides link together through covalent bonds between the sugar of one and the phosphate of the next, forming a sturdy sugar-phosphate backbone. This backbone provides the structural framework, while the bases protrude inward, ready to form connections.
Also, Read this Article in Detail: Three Main Parts of a Nucleotide: Structure and Functions.
The iconic double helix structure of DNA arises from two such polynucleotide chains winding around each other. These strands coil in a right-handed fashion, much like a twisted ladder. The bases from each strand pair up specifically: A always bonds with T via two hydrogen bonds, and G pairs with C through three hydrogen bonds. This complementary base pairing ensures that the strands fit together perfectly, maintaining a consistent width along the helix. Each full turn of the helix spans about 10 base pairs, with a distance of roughly 3.4 nanometers between turns.
What makes this structure even more ingenious is its antiparallel orientation. One strand runs from the 5′ end (where the phosphate is attached to the fifth carbon of the sugar) to the 3′ end, while the other runs in the opposite direction, from 3′ to 5′. This setup not only stabilizes the molecule but also facilitates replication. During cell division, the strands unzip, and each serves as a template for building a new complementary strand, ensuring genetic fidelity.
Beyond the basics, DNA’s grooves add another layer of functionality. The double helix features a major groove and a minor groove, which alternate along its length. The major groove is wider and deeper, exposing the edges of the base pairs for interactions with proteins like transcription factors. These proteins “read” the genetic code by binding to specific sequences, initiating processes such as protein synthesis. The minor groove, narrower and shallower, also plays a role in binding but is less sequence-specific.
Variations in DNA Forms: A, B, and Z Structures
While the right-handed double helix is the most common, DNA can adopt different conformations depending on environmental conditions like humidity, salt concentration, and sequence composition. These variations highlight DNA’s flexibility and adaptability.
The B-form DNA is the standard structure under normal physiological conditions. It’s a right-handed helix with about 10 base pairs per turn, a diameter of around 1.9 nanometers, and well-defined major and minor grooves. This form is hydrated and stable in cellular environments, making it ideal for storing genetic information.
In contrast, A-form DNA emerges in drier conditions or with certain sequences. It’s also right-handed but shorter and wider, with 11 base pairs per turn and a diameter of 2.3 nanometers. The base pairs tilt more, and the major groove becomes narrower and deeper, while the minor groove widens. This form is often seen in RNA-DNA hybrids or during transcription.
Then there’s Z-form DNA, a left-handed helix that forms in sequences with alternating purines and pyrimidines, like CG repeats. It’s elongated and thinner, with 12 base pairs per turn and a zig-zag backbone—hence the “Z” name. The major groove is flat, and the minor groove is deep and narrow. Z-DNA may play roles in gene regulation and is more common under high salt conditions.
To illustrate these differences, consider how they affect biological processes. For example, Z-DNA can influence chromatin structure, potentially activating or repressing genes in response to cellular stress.
Here’s a detailed comparison table of these DNA forms:
| Feature | A-Form DNA | B-Form DNA | Z-Form DNA |
|---|---|---|---|
| Helical Direction | Right-handed | Right-handed | Left-handed |
| Base Pairs per Turn | 11 | 10 | 12 |
| Helix Length per Turn | 2.8 nm | 3.4 nm | 4.56 nm |
| Diameter | 2.3 nm | 1.9 nm | 1.8 nm |
| Major Groove | Narrow and deep | Wide and shallow | Flat |
| Minor Groove | Wide and shallow | Narrow and deep | Narrow and deep |
| Base Tilt | Pronounced tilt | Slight tilt | Alternating syn/anti conformations |
| Preferred Conditions | Low hydration, purine-pyrimidine repeats | Physiological conditions | High salt, alternating sequences |
| Biological Role | Seen in hybrids, transcription | Standard genetic storage | Gene regulation, stress response |
This table underscores how subtle changes in structure can lead to significant functional differences, allowing DNA to respond to diverse cellular needs.
Physical Properties of DNA
DNA’s physical properties govern how it behaves in various environments, influencing everything from laboratory techniques to cellular packing.
One key property is solubility. DNA is highly polar due to its negatively charged phosphate groups in the backbone, making it readily soluble in water. This polarity arises from the ionic nature of the phosphates, which interact favorably with water molecules. However, adding salts or alcohols disrupts these interactions, causing DNA to precipitate out of solution—a principle used in DNA extraction protocols.
Another important aspect is ultraviolet absorption. The nitrogenous bases absorb UV light strongly at 260 nanometers, a feature exploited in spectrophotometry to quantify DNA. Double-stranded DNA absorbs less light than single-stranded because the bases are stacked and shielded in the helix. For instance, single-stranded DNA shows about 1.37 times the absorbance of its double-stranded counterpart at the same concentration.
Denaturation and renaturation are thermal properties that reveal DNA’s stability. Heating breaks the hydrogen bonds, unwinding the strands in a process called denaturation. The temperature at which half the DNA denatures, known as the melting temperature (Tm), depends on base composition: G-C pairs, with three hydrogen bonds, require higher temperatures (around 95°C for pure G-C regions) than A-T pairs (about 65°C). Upon cooling, complementary strands can renature, reforming the helix. This cycle is fundamental to techniques like polymerase chain reaction (PCR), where DNA is repeatedly denatured and annealed to amplify specific sequences.
Supercoiling adds another dimension to DNA’s physicality. In cells, DNA often twists beyond its relaxed state, forming supercoils that compact the molecule. Positive supercoiling tightens the helix, while negative supercoiling loosens it, aiding processes like transcription by making strands easier to separate. Enzymes called topoisomerases regulate this, preventing tangles in long DNA strands.
Consider the length of DNA: in a human cell, if stretched out, it measures about 2 meters. Supercoiling and wrapping around histone proteins into chromatin allow this to fit into a nucleus just microns wide. Without these properties, genetic material would be unmanageable.
Here are some additional physical properties in bullet points for clarity:
- Viscosity: DNA solutions are viscous due to the long, entangled strands; shearing can break them, reducing viscosity.
- Density: DNA has a buoyant density of about 1.7 g/cm³, varying with G-C content—higher G-C means denser DNA.
- Flexibility: Modeled as a semi-flexible rod, DNA bends with a persistence length of around 50 nm, influencing looping for gene regulation.
- Optical Activity: DNA exhibits circular dichroism, rotating plane-polarized light due to its helical asymmetry, useful in structural studies.
These properties make DNA a versatile molecule, adaptable to both cellular demands and experimental manipulations.
Chemical Properties of DNA: Polarity, Reactivity, and Base Interactions
Chemically, DNA’s properties stem from its molecular components and bonds, dictating its reactivity and interactions.
The polarity of DNA is primarily due to the phosphate groups, which carry a negative charge at physiological pH. This makes the backbone hydrophilic and repels other negatively charged molecules, while attracting positively charged proteins like histones for packaging.
Base stacking, driven by hydrophobic interactions and van der Waals forces, stabilizes the helix alongside hydrogen bonding. Purines (A, G) and pyrimidines (T, C) stack efficiently, contributing to the molecule’s rigidity.
DNA’s chemical stability is notable; it’s resistant to hydrolysis under normal conditions but can be damaged by UV radiation, chemicals, or enzymes. For example, UV light causes thymine dimers, leading to mutations if unrepaired.
In terms of composition, the base ratio follows Chargaff’s rules: A equals T, and G equals C, ensuring balance. Genomes vary in G-C content—from 20% in some parasites to over 70% in certain bacteria—affecting stability and evolution.
Examples abound in nature: thermophilic bacteria have high G-C content for heat resistance, while A-T rich regions in promoters facilitate unwinding for transcription.
A table comparing the nitrogenous bases:
| Base | Type | Structure | Hydrogen Bonds | Role in DNA |
|---|---|---|---|---|
| Adenine (A) | Purine | Two fused rings | 2 (with T) | Pairs with T; involved in energy transfer as ATP analog |
| Thymine (T) | Pyrimidine | Single ring | 2 (with A) | Pairs with A; protects against UV damage |
| Guanine (G) | Purine | Two fused rings | 3 (with C) | Pairs with C; signals in gene regulation |
| Cytosine (C) | Pyrimidine | Single ring | 3 (with G) | Pairs with G; prone to deamination mutations |
This table highlights how chemical differences influence pairing and function.
Functions and Biological Significance of DNA
DNA’s properties enable its primary functions: storing, replicating, and expressing genetic information.
As an information repository, the sequence of bases encodes genes, with triplets (codons) specifying amino acids. For humans, the genome spans 3 billion base pairs, housing about 20,000-25,000 genes.
Replication ensures inheritance: helicases unwind the helix, polymerases add nucleotides, and ligases seal strands, producing identical copies.
In expression, DNA transcribes into RNA, which translates into proteins. Properties like grooves and supercoiling regulate this, with enhancers looping to promoters.
DNA also varies by location: nuclear DNA is linear and diploid, while mitochondrial DNA is circular, haploid, and maternally inherited, encoding energy-related genes.
Biologically, DNA mutations drive evolution—point changes, insertions, or deletions alter traits. In medicine, understanding properties aids gene therapy, where edited DNA corrects disorders like cystic fibrosis.
For instance, CRISPR technology exploits base pairing to target sequences precisely, revolutionizing genetics.
In forensics, short tandem repeats (unique patterns) identify individuals, leveraging sequence variability.
Environmentally, DNA barcoding identifies species via conserved genes, aiding biodiversity studies.
These applications show DNA’s properties extending beyond biology into technology and society.
Advanced Insights: DNA in Extreme Conditions and Future Research
DNA thrives in diverse environments. In extremophiles, high G-C content withstands boiling temperatures, while in space simulations, it survives radiation, hinting at panspermia.
Chemically modified DNA, like with epigenetic marks (methylation), alters expression without sequence changes, influencing development and disease.
Future research explores synthetic DNA for data storage—its density could hold exabytes in grams—or xenonucleic acids with novel bases for therapeutics.
Challenges include ethical issues in editing, but properties like stability promise breakthroughs in personalized medicine.
In summary, DNA’s blend of structure and properties makes it life’s cornerstone, continually unveiling new wonders.
Read These Articles in Detail
- The Cell: The Fundamental Building Block of Life
- Prokaryotic Cells vs. Eukaryotic Cells: A Detailed Exploration
- Prokaryotic Cells: Definition, Anatomy, Functions, and Their Importance
- Eukaryotic Cells Explained: Definition, Structure, Functions, and Importance
- The Cell Membrane: Structure, Functions, and Evolutionary Significance
- The Cell Wall: Structure, Composition, Functions, and Evolutionary Significance
- Endomembrane System: A Detailed Exploration of the Cellular Machinery of Life
- Mitochondria: A Comprehensive Guide to Their Structure and Functions
- The Cell Envelope: The Protective Shield of Prokaryotic Cells
- Ribosomes: Definition, Structure, and Functions
- Endoplasmic Reticulum: Structure, Types, and Functions Explained
- The Golgi Apparatus: Structure, Functions, and Cellular Importance
- Cytoplasm and Nucleus: Structure, Functions, Differences, and Examples
- What Is Cell Signaling? Structure, Types, and Functions Explained
- Three Main Parts of a Nucleotide: Structure and Functions
- Enzymes: Definition, Structure, Classification, and Functions
- DNA: Structure, Function, Discovery, and Modern Applications
- DNA Replication: Steps, Process, and Genetic Importance
- Properties of DNA: Structure, Physical, and Chemical Characteristics
- DNA Packaging: How DNA Fits into the Nucleus of a Cell
- R-Factors and Plasmids: How Bacteria Develop Antibiotic Resistance
- Chromosomes: Structure, Types, Functions, and Role in Genetics
- Genetic Material: DNA, RNA, and Their Role in Heredity and Evolution
- Deoxyribose and Ribose: Structure and Function in DNA and RNA
- Differences Between Genes and DNA: Definitions, Structures, and Functions
- RNA: Definition, Structure, Types, Functions, and Its Role in Life
Frequently Asked Questions
FAQ 1: What is the basic structure of DNA, and how does it support its function?
DNA, or deoxyribonucleic acid, is a molecule that holds the genetic instructions for life. Its structure is both elegant and functional, enabling it to store and transmit genetic information effectively.
- Double Helix Formation: DNA consists of two long strands that twist into a double helix, resembling a twisted ladder. This shape, discovered in 1953 by James Watson and Francis Crick, provides stability and protects the genetic code inside.
- Nucleotide Building Blocks: Each strand is made of nucleotides, which include a sugar molecule (deoxyribose), a phosphate group, and one of four nitrogenous bases: adenine (A), thymine (T), cytosine (C), or guanine (G).
- Complementary Base Pairing: The bases pair specifically—A with T (via two hydrogen bonds) and C with G (via three hydrogen bonds)—forming the “rungs” of the ladder. This ensures accurate replication and information storage.
- Antiparallel Strands: The strands run in opposite directions (5′ to 3′ and 3′ to 5′), which is crucial for replication, as enzymes use each strand as a template to build a new one.
- Major and Minor Grooves: The helix has grooves where proteins can bind, enabling processes like transcription, where DNA’s code is read to produce proteins.
This structure allows DNA to compactly store vast amounts of information, replicate precisely during cell division, and interact with cellular machinery to express genes, making it the cornerstone of heredity.
FAQ 2: Why is DNA considered a polar molecule, and how does this affect its solubility?
DNA’s polarity is a key chemical property that influences how it behaves in different environments, particularly in terms of solubility.
- Phosphate-Sugar Backbone: The backbone of DNA, made of alternating sugar and phosphate groups, carries negative charges on the phosphate groups. These charges make DNA highly polar, meaning it interacts well with other polar molecules like water.
- Solubility in Water: Because water is also polar, DNA dissolves easily in aqueous environments, such as inside cells or in lab solutions. The negative charges attract water molecules, keeping DNA hydrated and functional.
- Effect of Salts and Alcohol: Adding salts (like sodium chloride) or alcohols (like ethanol) reduces DNA’s solubility. Salts neutralize the negative charges, and alcohol disrupts water interactions, causing DNA to precipitate. This is why alcohol is used in DNA extraction protocols.
- Biological Implications: Polarity ensures DNA remains in the watery environment of the cell nucleus or cytoplasm, where it can interact with enzymes and proteins for replication and gene expression.
This property is critical not only for DNA’s role in cells but also for laboratory techniques like DNA purification, where controlling solubility is essential.
FAQ 3: How does DNA absorb ultraviolet light, and why is this property useful?
DNA’s ability to absorb ultraviolet (UV) light is a fascinating physical property that has practical applications in science and research.
- Absorption at 260 nm: The nitrogenous bases (A, T, C, G) in DNA absorb UV light most strongly at a wavelength of 260 nanometers due to their aromatic ring structures. This absorption occurs because UV light excites electrons in the bases.
- Single vs. Double Strands: Single-stranded DNA absorbs about 1.37 times more UV light than double-stranded DNA at the same concentration. In the double helix, base stacking reduces exposure, lowering absorbance.
- Spectrophotometry Applications: Scientists use UV absorption to measure DNA concentration in a sample. A spectrophotometer shines UV light through a solution, and the amount of light absorbed at 260 nm indicates how much DNA is present.
- Purity Assessment: The ratio of absorbance at 260 nm to 280 nm helps assess DNA purity. A ratio of around 1.8 suggests pure DNA, while lower values may indicate protein contamination.
This property is invaluable in molecular biology labs, enabling researchers to quantify and verify DNA samples for experiments like sequencing or cloning.
FAQ 4: What are the different forms of DNA, and how do they differ?
DNA is not a one-size-fits-all molecule; it can adopt different structural forms depending on environmental conditions, each with unique characteristics.
- B-Form DNA: The most common form in cells, B-DNA is a right-handed helix with 10 base pairs per turn, a diameter of 1.9 nm, and wide, shallow major grooves. It’s stable under normal cellular conditions and ideal for genetic storage.
- A-Form DNA: Seen in low-hydration conditions or RNA-DNA hybrids, A-DNA is right-handed but shorter and wider (2.3 nm diameter, 11 base pairs per turn). Its major groove is narrower and deeper, suited for specific interactions during transcription.
- Z-Form DNA: A left-handed helix, Z-DNA forms in high-salt conditions or sequences with alternating purines and pyrimidines (e.g., CG repeats). It has 12 base pairs per turn, a thinner diameter (1.8 nm), and a flat major groove, potentially involved in gene regulation.
- Functional Roles: Each form has biological significance. B-DNA is the default for storage, A-DNA appears in temporary structures during transcription, and Z-DNA may regulate genes under stress.
These variations highlight DNA’s adaptability, allowing it to function in diverse cellular contexts and respond to environmental changes.
FAQ 5: What happens during DNA denaturation and renaturation?
Denaturation and renaturation are processes that reveal DNA’s dynamic nature, driven by its physical and chemical properties.
- Denaturation Process: When heated, the hydrogen bonds between base pairs (A-T, C-G) break, causing the double helix to unwind into two single strands. This typically occurs around the melting temperature (Tm), which varies based on sequence.
- Base Pair Influence: G-C pairs, held by three hydrogen bonds, require higher temperatures (around 95°C) to denature compared to A-T pairs (around 65°C) with two bonds. Thus, DNA with more G-C content has a higher Tm.
- Renaturation Process: Upon cooling, complementary strands can re-form hydrogen bonds and rewind into a double helix if their sequences align. This process is slower and depends on proper conditions like temperature and salt concentration.
- Applications in Science: Denaturation and renaturation are central to techniques like PCR (polymerase chain reaction), where DNA is heated to separate strands and cooled to allow primers to bind for amplification.
These processes demonstrate DNA’s ability to maintain and recover its structure, crucial for both cellular functions and laboratory manipulations.
FAQ 6: How does DNA’s supercoiling affect its function in cells?
Supercoiling refers to the twisting of DNA beyond its relaxed double-helical state, a property that significantly impacts its biological roles.
- Types of Supercoiling: DNA can be positively supercoiled (overwound, tighter helix) or negatively supercoiled (underwound, looser helix). Negative supercoiling is more common in cells as it facilitates strand separation.
- Role in Compaction: Supercoiling compacts DNA, allowing the 2 meters of DNA in a human cell to fit into a tiny nucleus. It’s further organized by wrapping around histone proteins to form chromatin.
- Facilitating Processes: Negative supercoiling reduces the energy needed to unwind DNA during replication and transcription, making it easier for enzymes like polymerases to access the genetic code.
- Regulation by Enzymes: Topoisomerases are enzymes that manage supercoiling by cutting and resealing DNA strands, preventing tangles and maintaining proper tension.
Supercoiling is essential for DNA’s packaging and accessibility, ensuring efficient storage and use of genetic information in cells.
FAQ 7: How do the nitrogenous bases contribute to DNA’s genetic function?
The nitrogenous bases (adenine, thymine, cytosine, guanine) are the heart of DNA’s ability to store and transmit genetic information.
- Encoding Information: The sequence of bases forms the genetic code, with groups of three bases (codons) specifying amino acids that build proteins. For example, the codon AUG codes for methionine.
- Complementary Pairing: A pairs with T, and G pairs with C, ensuring accurate replication and transcription. This specificity preserves the genetic information across generations.
- Stability and Variability: G-C pairs, with three hydrogen bonds, are stronger than A-T pairs, affecting DNA’s stability. Genomes with higher G-C content are more resistant to denaturation, as seen in thermophilic bacteria.
- Functional Diversity: Bases also play roles beyond coding. Guanine, for instance, forms structures like G-quadruplexes that regulate gene expression in telomeres.
The precise arrangement and pairing of these bases make DNA a robust yet flexible medium for genetic information.
FAQ 8: Why is DNA’s stability important, and how is it maintained?
DNA’s stability is critical for preserving genetic information, but it must also allow controlled changes for processes like replication.
- Chemical Stability: The sugar-phosphate backbone resists hydrolysis under normal conditions, protecting the genetic code. However, it can be damaged by UV light or chemicals, forming mutations like thymine dimers.
- Base Pairing Strength: The hydrogen bonds and base stacking (via hydrophobic interactions) keep the double helix intact. G-C-rich regions are particularly stable due to stronger bonding.
- Repair Mechanisms: Cells have enzymes like DNA polymerases and repair proteins that fix damage, such as mismatches or breaks, ensuring fidelity during replication.
- Environmental Adaptations: In extremophiles, high G-C content enhances stability in harsh conditions like high temperatures, as seen in bacteria living in hot springs.
This balance of stability and flexibility allows DNA to maintain genetic integrity while supporting essential cellular functions.
FAQ 9: How does DNA’s structure enable its use in biotechnology?
DNA’s unique properties make it a cornerstone of modern biotechnology, from medical research to forensic science.
- Replication Precision: The complementary base pairing allows techniques like PCR to amplify specific DNA sequences for analysis, used in diagnostics and research.
- Sequence Specificity: Tools like CRISPR exploit base pairing to edit genes precisely, correcting mutations in diseases like sickle cell anemia.
- UV Absorption and Quantification: DNA’s absorbance at 260 nm enables accurate measurement in labs, critical for sequencing or cloning experiments.
- Forensic Applications: Short tandem repeats (STRs) in DNA vary between individuals, enabling identification in criminal investigations or paternity testing.
These applications leverage DNA’s structural and chemical properties to advance science and solve real-world problems.
FAQ 10: What is the significance of DNA’s grooves, and how do they function in cells?
The major and minor grooves of DNA’s double helix are critical for its interactions with cellular machinery.
- Structural Features: The major groove is wide and deep, exposing base pair edges for specific binding by proteins. The minor groove is narrower, allowing less specific interactions.
- Protein Binding: Transcription factors bind to the major groove to recognize specific DNA sequences, initiating gene expression. For example, the TATA-binding protein targets promoter regions to start transcription.
- Regulatory Roles: The grooves allow DNA to interact with enzymes and regulatory proteins, controlling processes like replication and repair.
- Drug Interactions: Some drugs, like chemotherapy agents, bind to grooves to interfere with DNA function in cancer cells, highlighting their therapeutic importance.
The grooves make DNA accessible to the cellular machinery, enabling precise regulation of genetic processes.
FAQ 11: What are the detailed steps involved in DNA replication?
DNA replication is a vital process where a cell duplicates its genetic material before division, ensuring each new cell gets an identical copy. This intricate mechanism involves several enzymes and occurs in a semi-conservative manner, meaning each new DNA molecule consists of one original strand and one newly synthesized strand.
- Initiation Phase: The process begins at specific sites called origins of replication. Here, the enzyme helicase unwinds the double helix by breaking the hydrogen bonds between base pairs, creating a replication fork. Single-strand binding proteins then coat the exposed strands to prevent them from reannealing, while topoisomerases relieve the tension from unwinding by cutting and resealing the DNA ahead of the fork.
- Primer Binding: DNA polymerase, the key enzyme for synthesis, requires a short RNA primer to start adding nucleotides. Primase synthesizes these primers on both strands. On the leading strand, which is oriented 5′ to 3′ toward the fork, synthesis is continuous. On the lagging strand, oriented 3′ to 5′, primers are laid down in segments.
- Elongation: DNA polymerase III extends the primers by adding deoxyribonucleotides complementary to the template strand, following base-pairing rules (A with T, G with C). On the leading strand, elongation proceeds smoothly. On the lagging strand, it occurs in short Okazaki fragments, each starting from a new primer. DNA polymerase I later replaces the RNA primers with DNA, and ligase seals the gaps between fragments.
- Termination: Replication continues until the entire molecule is copied. In prokaryotes, it ends when forks meet; in eukaryotes, telomeres at chromosome ends are handled by telomerase to prevent shortening. Proofreading by DNA polymerase corrects errors, maintaining high fidelity with an error rate of about one in a billion bases.
- Post-Replication Checks: After synthesis, mismatch repair systems scan for and fix any remaining errors, ensuring genetic stability. This step is crucial for preventing mutations that could lead to diseases like cancer.
Understanding these steps highlights how DNA’s structure, with its antiparallel strands and complementary bases, enables precise copying essential for life.
FAQ 12: How do epigenetic modifications affect DNA without changing its sequence?
Epigenetic modifications are fascinating chemical changes that influence how genes are expressed without altering the underlying DNA sequence. These modifications act like switches, turning genes on or off in response to environmental factors, development needs, or even lifestyle choices. For instance, they play a key role in cell differentiation, where a stem cell becomes a neuron or a muscle cell, all while keeping the same genetic code. This layer of regulation adds complexity to genetics, explaining why identical twins might develop different traits over time despite sharing the same DNA.
One primary type is DNA methylation, where a methyl group attaches to cytosine bases, often in promoter regions. This typically silences gene expression by making the DNA less accessible to transcription machinery. In contrast, histone modifications involve adding or removing groups like acetyl or methyl to histone proteins around which DNA wraps. Acetylation loosens the chromatin structure, promoting gene activation, while deacetylation tightens it, leading to repression. Non-coding RNAs also contribute by guiding these changes or directly interfering with gene expression. These mechanisms are reversible, allowing cells to adapt dynamically to signals like stress or nutrition.
The implications extend to health and disease. Aberrant epigenetic patterns are linked to conditions such as cancer, where hypermethylation might shut off tumor suppressor genes, or neurological disorders like Alzheimer’s, influenced by altered histone marks. Research shows that factors like diet, exercise, and exposure to toxins can induce these modifications, potentially passing them to offspring through mechanisms like sperm or egg epigenomes. This transgenerational effect underscores epigenetics’ role in evolution and adaptation, beyond traditional mutations.
In biotechnology, understanding epigenetics opens doors to therapies. Drugs like HDAC inhibitors target histone modifications to reactivate silenced genes in cancer treatment. Future applications might include personalized medicine, where epigenetic profiles guide preventive strategies. Overall, these modifications reveal that our genome is not a fixed blueprint but a responsive system shaped by both nature and nurture.
FAQ 13: What are the main types of DNA damage and their corresponding repair mechanisms?
DNA is constantly under assault from internal and external factors, leading to various forms of damage that, if unrepaired, can cause mutations or cell death. Fortunately, cells have evolved sophisticated repair pathways to maintain genomic integrity. Below is a comprehensive table outlining key types of DNA damage, their causes, and the primary repair mechanisms involved.
| Type of DNA Damage | Common Causes | Repair Mechanism | How It Works |
|---|---|---|---|
| Base Modifications | Oxidative stress from reactive oxygen species (ROS), alkylation by chemicals | Base Excision Repair (BER) | Glycosylases remove damaged bases, creating an abasic site; AP endonuclease cleaves the backbone, and polymerase fills the gap, sealed by ligase. |
| Mismatches | Replication errors, like incorrect base insertion | Mismatch Repair (MMR) | Proteins like MutS recognize mismatches; the incorrect strand is excised, and polymerase resynthesizes the correct sequence. |
| Bulky Adducts | UV radiation forming thymine dimers, chemical carcinogens | Nucleotide Excision Repair (NER) | Damage is detected by proteins; a segment of about 28 nucleotides is removed, and the gap is filled using the intact strand as a template. |
| Double-Strand Breaks | Ionizing radiation, replication fork collapse | Non-Homologous End Joining (NHEJ) or Homologous Recombination (HR) | NHEJ directly ligates broken ends (error-prone); HR uses a sister chromatid as a template for accurate repair during S/G2 phases. |
| Crosslinks | Chemicals like chemotherapy drugs, UV light | Interstrand Crosslink Repair (ICL) | Involves NER and HR; Fanconi anemia pathway coordinates unhooking and repair of crosslinked strands. |
| Single-Strand Breaks | Oxidative damage, enzymatic activity | Single-Strand Break Repair (SSBR) | Similar to BER; PARP proteins detect breaks, and repair enzymes fill and seal the nick. |
This table illustrates the diversity of threats to DNA and the tailored responses that safeguard our genetic material, crucial for preventing diseases like cancer.
FAQ 14: What are the key differences between nuclear DNA and mitochondrial DNA?
Nuclear DNA and mitochondrial DNA both carry genetic information but differ significantly in structure, function, and inheritance, reflecting their distinct roles in cellular processes.
- Location and Structure: Nuclear DNA resides in the cell’s nucleus, organized into linear chromosomes wrapped around histones, forming chromatin. In humans, it consists of 46 chromosomes (23 pairs) totaling about 3 billion base pairs. Mitochondrial DNA, however, is located in the mitochondria, the cell’s energy producers, and is circular, lacking histones, with only about 16,500 base pairs encoding 37 genes.
- Inheritance Pattern: Nuclear DNA is inherited from both parents, with half from each, allowing for genetic recombination. Mitochondrial DNA is maternally inherited, passed only through the egg cell, as sperm mitochondria are typically degraded after fertilization. This makes it useful for tracing maternal lineages.
- Gene Content and Function: Nuclear DNA encodes the vast majority of genes (around 20,000-25,000), covering diverse functions like development and metabolism. Mitochondrial DNA encodes primarily for proteins involved in oxidative phosphorylation, essential for ATP production, plus tRNAs and rRNAs.
- Replication and Repair: Nuclear DNA replicates once per cell cycle with robust repair mechanisms. Mitochondrial DNA replicates independently, often multiple times, but has fewer repair pathways, making it more prone to mutations over time.
- Mutation Rate and Implications: Mitochondrial DNA mutates faster due to proximity to ROS from energy production, contributing to aging and diseases like mitochondrial myopathies. Nuclear DNA’s lower mutation rate supports stable inheritance but can lead to broader genetic disorders when altered.
These differences highlight how mitochondrial DNA supports energy needs while nuclear DNA handles overall cellular governance.
FAQ 15: How is DNA used in forensic science to solve crimes?
In forensic science, DNA serves as a powerful tool for identifying individuals and linking them to crime scenes, revolutionizing investigations since its first use in the 1980s. The process begins with collecting biological evidence like blood, saliva, hair, or semen from a scene. This evidence contains cells with DNA, which forensic experts extract and analyze to create a unique profile. Unlike fingerprints, which can be smudged, DNA is more resilient and can be recovered from tiny samples, even degraded ones, making it invaluable for cold cases.
The core technique is DNA profiling, often using short tandem repeats (STRs)—repeating sequences that vary greatly between people. Analysts amplify these regions via polymerase chain reaction (PCR) and compare them against suspect samples or databases like CODIS. A match can confirm a suspect’s presence at a scene with high probability, sometimes one in a quadrillion. This has led to convictions in assaults and murders, as well as exonerations for the wrongly accused through post-conviction testing.
Beyond identification, DNA helps reconstruct events. Touch DNA from skin cells on weapons or familial searching in databases can identify relatives of unknown suspects. In mass disasters, it aids victim identification when other methods fail. However, challenges like contamination or mixed samples require careful handling and advanced techniques like next-generation sequencing for complex mixtures.
Ethically, DNA use raises privacy concerns with expanding databases, but its accuracy has transformed justice, ensuring more reliable outcomes in courts worldwide.
FAQ 16: What is synthetic DNA, and what are its potential applications?
Synthetic DNA refers to artificially created deoxyribonucleic acid sequences designed and assembled in laboratories, rather than extracted from living organisms. This technology allows scientists to build custom genetic material for research and practical uses, expanding beyond natural limitations.
- Definition and Creation: Synthetic DNA is produced through chemical synthesis, where nucleotides are linked to form desired sequences. Methods like oligonucleotide synthesis enable precise control, creating genes or entire genomes not found in nature.
- Applications in Medicine: It’s used to develop vaccines, gene therapies, and diagnostics. For example, synthetic sequences can produce proteins for treating genetic disorders or engineer viruses for targeted cancer therapies.
- Biotechnology and Agriculture: In biofuels, microbes with synthetic DNA convert waste into energy. In farming, it creates pest-resistant crops or enhances nutritional value, improving yields sustainably.
- Data Storage and Computing: DNA’s density makes it ideal for archiving data; synthetic strands encode information in base sequences, potentially storing exabytes in grams.
- Environmental and Industrial Uses: Synthetic DNA engineers bacteria for bioremediation, breaking down pollutants, or produces eco-friendly materials like spider silk proteins.
These applications demonstrate synthetic DNA’s versatility in solving global challenges.
FAQ 17: What are the major DNA sequencing technologies available today?
DNA sequencing has evolved from labor-intensive methods to high-throughput technologies, enabling rapid genome analysis. The table below compares key technologies, their principles, advantages, and applications.
| Technology | Principle | Key Advantages | Limitations | Common Applications |
|---|---|---|---|---|
| Sanger Sequencing | Chain-termination with dideoxynucleotides | High accuracy (99.99%), long reads (up to 1,000 bp) | Low throughput, time-consuming | Small-scale projects, validation of NGS |
| Next-Generation Sequencing (NGS) | Massively parallel sequencing of short fragments | High throughput, cost-effective for large genomes | Short reads (100-300 bp), error-prone in repeats | Whole-genome sequencing, transcriptomics |
| Illumina Sequencing | Reversible terminator chemistry on flow cells | Massive data output, low cost per base | Requires amplification, potential biases | Clinical diagnostics, metagenomics |
| PacBio Long-Read | Single-molecule real-time (SMRT) sequencing | Long reads (10-20 kb), resolves complex regions | Higher error rate (corrected by circular consensus) | De novo assembly, structural variants |
| Oxford Nanopore | Nanopore threading detects base changes | Ultra-long reads (>1 Mb), portable devices | Variable accuracy, improving with updates | Field-based sequencing, rapid pathogen ID |
These technologies drive advancements in personalized medicine and research.
FAQ 18: How do DNA mutations contribute to the process of evolution?
DNA mutations are changes in the genetic sequence that introduce variation, serving as the raw material for evolution. Without them, populations would lack the diversity needed to adapt to changing environments. Most mutations are neutral or harmful, but beneficial ones can spread through natural selection, leading to evolutionary changes over generations. For example, a mutation might alter a protein’s function, improving an organism’s survival, like antibiotic resistance in bacteria.
Types of mutations include point mutations, insertions, deletions, and duplications, each potentially affecting gene expression or protein structure. In evolution, these accumulate, driving speciation. The peppered moth’s color change during the Industrial Revolution exemplifies how mutations for darker pigmentation became advantageous in polluted areas, increasing their frequency.
Mutations also interact with other forces like genetic drift and gene flow. In small populations, drift can fix neutral mutations randomly. Overall, they enable adaptation, from human lactose tolerance to viral evasions of immunity, shaping biodiversity.
FAQ 19: How do DNA vaccines work to protect against diseases?
DNA vaccines represent an innovative approach to immunization, using genetic material to trigger immune responses against pathogens.
- Delivery Mechanism: The vaccine consists of a plasmid—a small, circular DNA molecule—encoding a pathogen’s antigen, like a viral protein. It’s injected into muscle or skin, where cells take it up.
- Cellular Uptake and Expression: Host cells transcribe the DNA into mRNA, then translate it into the antigen protein. This mimics natural infection without causing disease.
- Immune Activation: The produced antigen is presented on cell surfaces, activating T cells for cellular immunity and B cells for antibody production, providing long-term protection.
- Advantages Over Traditional Vaccines: DNA vaccines are stable, easy to produce, and can elicit both humoral and cellular responses, effective against viruses like COVID-19 or cancers.
- Safety and Enhancements: They don’t integrate into the genome significantly, reducing risks; electroporation or adjuvants improve uptake for stronger immunity.
This technology offers rapid development for emerging threats.
FAQ 20: What are the future trends in DNA research and technology?
Emerging trends in DNA research promise transformative impacts across fields. The table below summarizes key developments, their drivers, and potential outcomes.
| Trend | Key Drivers | Potential Outcomes |
|---|---|---|
| Advanced Gene Editing | CRISPR enhancements, base and prime editing | Precise therapies for genetic diseases, ethical debates on germline edits. |
| AI in Genomics | Machine learning for data analysis | Faster variant interpretation, personalized medicine predictions. |
| Synthetic Biology Expansion | Automated DNA synthesis, circuit design | Custom organisms for biofuels, sustainable materials. |
| Long-Read Sequencing | Nanopore and PacBio improvements | Better assembly of complex genomes, real-time diagnostics. |
| Epigenomics and Multi-Omics | Integration of epigenetics with other data | Insights into aging, disease prevention strategies. |
| DNA Data Storage | High-density encoding needs | Archival solutions for big data, long-term preservation. |
These trends signal a future where DNA tech addresses global challenges.
Acknowledgement
The educational website Examsmeta.com sincerely expresses its gratitude to the scientific community and various reputable sources that have significantly enriched the article “Properties of DNA: Structure, Physical, and Chemical Characteristics.” The insights and data provided by these platforms have been instrumental in ensuring the accuracy and depth of the information presented. Their comprehensive resources on DNA’s structure, properties, and applications have allowed us to craft a detailed and informative piece for our readers.
Below, acknowledges the key sources that contributed to this article:
- National Human Genome Research Institute (NHGRI): For providing authoritative information on DNA structure, replication, and genomic research, which formed the backbone of our explanations.
- Nature Education (Nature): For offering detailed insights into DNA’s physical and chemical properties, including supercoiling and epigenetic modifications.
- ScienceDirect (ScienceDirect): For supplying peer-reviewed data on DNA repair mechanisms, sequencing technologies, and synthetic biology applications.
- PubMed (PubMed): For facilitating access to scientific studies on DNA mutations, epigenetics, and their roles in evolution and disease.
- Encyclopaedia Britannica (Britannica): For providing clear, foundational knowledge on DNA’s molecular structure and biological significance.

