Reactive oxygen species, often abbreviated as ROS, are fascinating molecules that play a pivotal role in our bodies. Produced naturally during everyday cellular activities like metabolism, these species can be both helpful and harmful. On one hand, they act as signaling molecules that help regulate important processes, keeping our cells functioning smoothly. On the other, when they build up too much, they can cause damage leading to oxidative stress, which is linked to aging and various diseases.
This article dives deep into the world of ROS, exploring their types, how they’re made, the body’s defenses against them, and their complex relationship with aging. We’ll look at scientific insights, real-world examples, and even some challenges to long-held theories, all explained in straightforward terms.
Table of Contents
Imagine your body as a bustling factory where energy is constantly being produced. In this factory, ROS are like the sparks from machinery – necessary for some operations but dangerous if not controlled. Scientists have studied these molecules for decades, starting with ideas from the mid-20th century, and recent research continues to reveal their nuanced effects. Whether you’re curious about why we age or how to maintain better health, understanding ROS offers valuable clues.
What Are Reactive Oxygen Species?
Reactive Oxygen Species (ROS) are chemically reactive molecules containing oxygen. They form as byproducts when our cells convert food into energy, a process happening in tiny powerhouses called mitochondria. Not all ROS are the same; some are free radicals with unpaired electrons, making them eager to react with other molecules, while others are non-radical but still oxidizing.
Think of ROS as a family of compounds, each with unique properties. For instance, they can influence cell behavior in small amounts but wreak havoc in excess. This duality means referring to them as a single group can sometimes oversimplify things – different ROS might trigger specific signals or damages depending on where they are in the cell, how long they’re around, and their concentration.
From a broader perspective, ROS aren’t just troublemakers. They’re essential for life, involved in everything from fighting infections to cell communication. However, imbalances can tip the scale toward problems like inflammation or tissue damage.
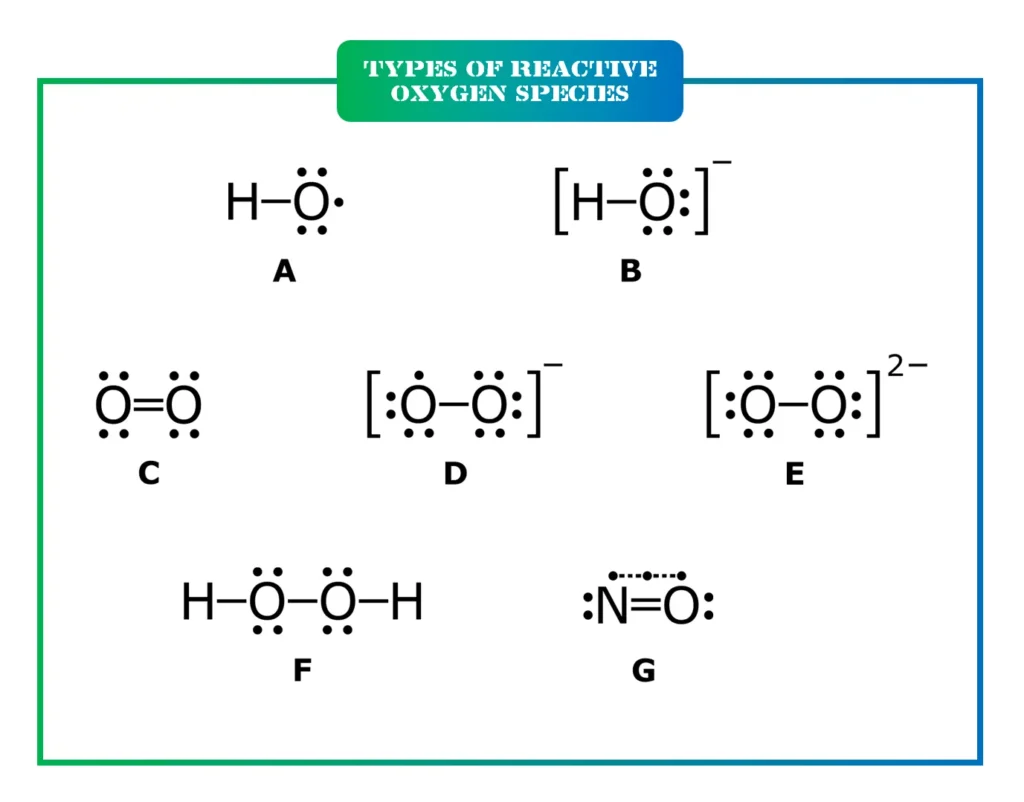
Types of Reactive Oxygen Species
There are several key types of ROS, each with distinct characteristics and roles. Here’s a breakdown to make it clearer:
- Superoxide anion (O₂⁻): This is often the first ROS produced in cells. It’s a free radical generated during oxygen reduction and can signal for immune responses but also contribute to stress if not managed.
- Hydrogen peroxide (H₂O₂): Not a free radical, but highly reactive. It acts as a messenger in low levels, helping cells adapt to stress, but high amounts can lead to damage.
- Hydroxyl radical (HO•): The most potent and reactive ROS. It’s short-lived and attacks nearby molecules like DNA or proteins, causing immediate harm.
- Peroxyl radicals (ROO•): These include hydroperoxyl (HOO•) and are involved in lipid peroxidation, where fats in cell membranes break down.
- Nitric oxide (•NO): A gas that’s crucial for blood vessel relaxation and immune function, but it can form harmful derivatives like peroxynitrite.
- Peroxynitrite (ONOO⁻): Formed from superoxide and nitric oxide, it’s a strong oxidant linked to protein and DNA damage.
- Hypochlorous acid (HOCl): Produced by immune cells to kill pathogens, but excess can harm healthy tissues.
Other related species include singlet oxygen and ozone, though they’re less common in biological contexts. Each type interacts differently, highlighting why ROS can’t be lumped together.
To visualize this variety, consider the following table summarizing major ROS types, their radical status, and primary effects:
| Type of ROS | Radical Status | Key Properties | Biological Role | Potential Harm |
|---|---|---|---|---|
| Superoxide anion (O₂⁻) | Free radical | Produced by one-electron reduction of O₂ | Signaling in immunity and stress response | Reacts to form more damaging species |
| Hydrogen peroxide (H₂O₂) | Non-radical | Diffuses through membranes via channels | Second messenger in cell adaptation | Leads to oxidative damage if accumulated |
| Hydroxyl radical (HO•) | Free radical | Extremely reactive, short half-life | Not typically a signal; mostly damaging | Attacks DNA, proteins, lipids instantly |
| Peroxyl radicals (ROO•) | Free radical | Involved in chain reactions | Lipid peroxidation initiation | Generates toxic byproducts like aldehydes |
| Nitric oxide (•NO) | Free radical | Gasotransmitter, diffuses easily | Vasodilation, anti-inflammatory | Forms peroxynitrite in excess |
| Peroxynitrite (ONOO⁻) | Non-radical | Highly oxidizing, reacts with CO₂ | Limited signaling; mostly pathological | Causes nitration of proteins |
| Hypochlorous acid (HOCl) | Non-radical | Produced by myeloperoxidase | Antimicrobial in immune response | Damages tissues in chronic inflammation |
| Singlet oxygen (¹O₂) | Non-radical | Excited oxygen state | Photodynamic therapy applications | Membrane and DNA damage |
| Ozone (O₃) | Non-radical | Environmental pollutant | Rare in biology; signaling in plants | Respiratory irritation and cell death |
This table shows how diverse ROS are, with some like H₂O₂ balancing between helpful and harmful more than others.
How Are Reactive Oxygen Species Produced?
ROS production happens through various pathways, mainly as side effects of normal metabolism. The primary site is the mitochondria during electron transport chain activity, where oxygen accepts electrons but sometimes forms superoxide instead of water.
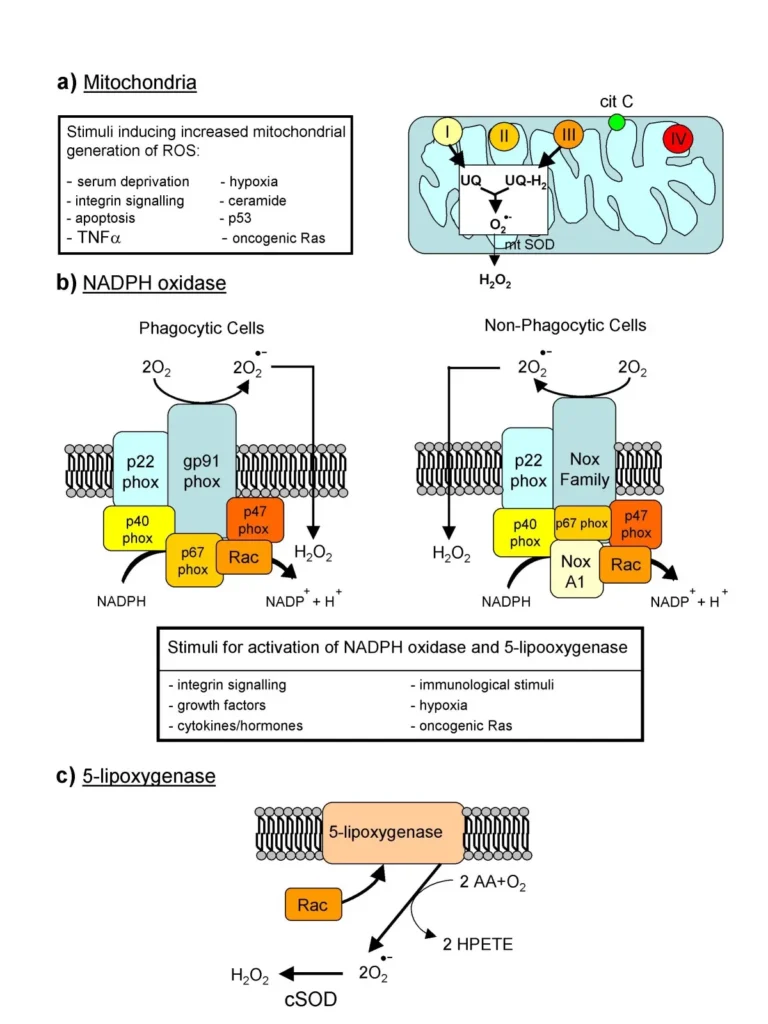
Enzymatic sources include:
- NADPH oxidases (NOX enzymes): These are a family of seven isoforms (NOX1 to NOX5, DUOX1, DUOX2) located in cell membranes. They deliberately produce ROS for defense against pathogens or signaling.
- Xanthine oxidase: Converts hypoxanthine to uric acid, releasing superoxide.
- Cytochrome P450 enzymes: Involved in drug metabolism, generating ROS as byproducts.
Non-enzymatic production occurs via reactions like the Fenton reaction, where iron catalyzes H₂O₂ breakdown into hydroxyl radicals.
Here’s a key reaction sequence for primary ROS formation:
$$[ O_2 + e^- \rightarrow O_2^{\bullet-} + e^- (+2H^+) \leftrightarrow H_2O_2 + e^- \rightarrow HO^{\bullet} + HO^- + e^- (+H^+) \leftrightarrow 2H_2O ]$$
This stepwise reduction of oxygen illustrates how superoxide leads to hydrogen peroxide and then hydroxyl radical.
Another important one is the Haber-Weiss reaction:
$$[ O_2^{\bullet-} + H_2O_2 \rightarrow HO^{\bullet} + HO^- + O_2 ]$$
With the Fenton step:
$$[ Fe^{3+} + O_2^{\bullet-} \rightarrow Fe^{2+} + O_2 ]$$
$$[ Fe^{2+} + H_2O_2 \rightarrow Fe^{3+} + HO^{\bullet} + HO^- ]$$
External factors like UV radiation, pollution, or smoking can also boost ROS production, exacerbating internal sources.
In plants and animals alike, ROS arise from similar mechanisms, but in humans, mitochondrial leakage accounts for about 1-2% of oxygen consumption turning into ROS.
The Body’s Antioxidant Defenses
To counter ROS, our bodies have a sophisticated antioxidant system, acting like a shield against oxidative damage. This includes enzymes, proteins, and small molecules that neutralize ROS or repair their harm.
Enzymatic antioxidants form the first line:
- Superoxide dismutase (SOD): Converts superoxide to hydrogen peroxide. There are three forms: SOD1 (cytoplasmic), SOD2 (mitochondrial), SOD3 (extracellular).
- Catalase (CAT): Breaks down hydrogen peroxide into water and oxygen, mainly in peroxisomes.
- Glutathione peroxidase (GPx): Uses glutathione to reduce peroxides, protecting lipids.
Non-enzymatic antioxidants include:
- Vitamins: Vitamin C (ascorbic acid) scavenges radicals in water-soluble areas; vitamin E (tocopherol) protects lipids.
- Glutathione (GSH): A tripeptide that donates electrons to neutralize ROS.
- Carotenoids and flavonoids: Plant-derived compounds with antioxidant properties.
These defenses work in tiers. For example, SOD handles superoxide, then CAT or GPx manages the resulting peroxide. If overwhelmed, oxidative stress ensues, damaging cells.
In a healthy state, this balance maintains redox homeostasis. However, aging or disease can deplete antioxidants, leading to issues. Studies show that boosting these defenses, like through diet rich in fruits and veggies, can mitigate some effects.
Consider this detailed table on antioxidant defenses:
| Antioxidant Type | Examples | Mechanism of Action | Location in Cell | Role in Health |
|---|---|---|---|---|
| Enzymatic – Primary | Superoxide dismutase (SOD) | Dismutates O₂⁻ to H₂O₂ and O₂ | Cytoplasm, mitochondria, extracellular | Prevents superoxide accumulation |
| Enzymatic – Secondary | Catalase (CAT) | Decomposes H₂O₂ to H₂O and O₂ | Peroxisomes | Handles high H₂O₂ levels in liver |
| Enzymatic – Tertiary | Glutathione peroxidase (GPx) | Reduces peroxides using GSH | Cytoplasm, mitochondria | Protects against lipid peroxidation |
| Non-enzymatic – Water-soluble | Vitamin C (ascorbic acid) | Donates electrons to radicals | Cytosol, extracellular fluid | Regenerates vitamin E, immune support |
| Non-enzymatic – Lipid-soluble | Vitamin E (tocopherol) | Breaks lipid peroxidation chains | Cell membranes | Guards against fatty acid damage |
| Non-enzymatic – Thiol-based | Glutathione (GSH) | Reduces disulfides and peroxides | All cellular compartments | Master antioxidant, detoxifies xenobiotics |
| Non-enzymatic – Plant-derived | Beta-carotene, flavonoids | Quench singlet oxygen and radicals | Membranes, cytosol | Anti-inflammatory, cancer prevention |
| Repair Enzymes | Methionine sulfoxide reductase | Repairs oxidized proteins | Cytoplasm | Restores protein function post-damage |
| Metal-binding Proteins | Ferritin, ceruloplasmin | Sequesters iron and copper | Cytoplasm, plasma | Prevents Fenton reaction catalysis |
| Transcription Factors | Nrf2 pathway activators | Upregulates antioxidant genes | Nucleus | Adaptive response to stress |
This table highlights the multi-layered protection, showing how enzymes and molecules collaborate.
The Dual Role of ROS: Signaling Versus Damage
ROS aren’t villains; they’re double agents. At low levels, known as oxidative eustress, they facilitate cellular signaling. For example, H₂O₂ activates pathways like MAPK or NF-κB, promoting cell growth, differentiation, and survival.
In immunity, ROS from NOX enzymes help white blood cells kill invaders. In blood vessels, •NO relaxes muscles for better flow.
But when ROS exceed defenses, oxidative stress hits. This damages biomolecules:
- DNA: Leads to mutations, cancer risk.
- Proteins: Alters structure, impairs function.
- Lipids: Peroxidation creates toxic products like MDA.
This shift from signal to stressor depends on dose and duration. Recent research emphasizes ROS as regulators, not just destroyers.
For instance, in exercise, mild ROS boosts muscle adaptation, but overtraining causes fatigue.
ROS and the Free Radical Theory of Aging
Back in 1956, Denham Harman proposed the free radical theory, suggesting ROS from metabolism drive aging by accumulating damage. This idea sparked research linking ROS to wrinkled skin, weakened muscles, and cognitive decline.
However, updates challenge it. Long-lived animals like naked mole rats have high ROS but robust defenses. Genetic tweaks increasing ROS sometimes extend lifespan, suggesting adaptive roles.
The theory evolved to focus on mitochondrial DNA damage, where ROS mutations impair energy production, creating a vicious cycle.
Critics argue ROS mediate stress responses that combat aging damage, not cause it outright. It’s now seen as part of a bigger picture, including inflammation and protein mishandling.
Molecular Aspects of ROS in Aging
At the molecular level, ROS oxidize proteins, forming carbonyls or cross-links that hinder function. They also modify DNA bases, leading to errors in replication.
In signaling, ROS target cysteines in proteins, creating reversible modifications like disulfides for regulation.
Aging sees rising ROS from declining mitochondrial efficiency, amplifying damage. For example, oxidized lipids like 4-HNE bind proteins, disrupting enzymes.
Cellular Aspects of ROS in Aging
Cells use ROS for decisions like apoptosis (programmed death) or autophagy (recycling). In senescence, persistent ROS halts division, contributing to tissue aging.
Mitochondria are central; damaged ones leak more ROS, spreading stress. Stem cells, vital for repair, suffer ROS-induced exhaustion with age.
Examples: In neurons, ROS aid synaptic plasticity but excess links to neurodegeneration.
Physiological Aspects of ROS in Aging
System-wide, ROS affect organs differently. In the heart, they contribute to stiffening arteries; in the brain, to plaque buildup.
Aging reduces antioxidant capacity, tilting balance. Exercise or calorie restriction can enhance defenses, lowering ROS effects.
In reproduction, sperm ROS aid fertilization but excess causes infertility.
ROS in Age-Related Diseases: Real-World Examples
ROS feature prominently in diseases like:
- Cardiovascular disease: Excess ROS oxidizes LDL, forming plaques. Peroxynitrite impairs vessel function.
- Neurodegenerative disorders: In Alzheimer’s, ROS promote amyloid aggregation; in Parkinson’s, they damage dopamine neurons.
- Cancer: ROS mutate DNA but also kill cancer cells in therapy.
- Diabetes: High glucose boosts ROS, damaging insulin-producing cells.
- Age-related macular degeneration: ROS harm retinal cells via mitochondrial DNA damage.
These examples show ROS as contributors, not sole causes, often intertwined with inflammation.
To sum up key disease links:
- Cardiovascular: Endothelial dysfunction from ONOO⁻.
- Neuro: Oxidative damage in amyloid and tau proteins.
- Metabolic: Insulin resistance via ROS-activated pathways.
- Ocular: Photoreceptor loss from UV-induced ROS.
Strategies to Manage ROS and Promote Healthy Aging
While we can’t eliminate ROS, lifestyle choices help. Antioxidant-rich diets (berries, nuts) support defenses. Regular exercise induces mild ROS for adaptation.
Supplements like coenzyme Q10 or resveratrol show promise in studies, though results vary.
Future therapies might target specific ROS sources, like NOX inhibitors for inflammation.
In conclusion, ROS embody the complexity of biology – essential yet risky. By understanding their dual nature, we can better navigate aging and health. Research continues to unfold their secrets, offering hope for interventions that extend not just lifespan, but healthspan.
Frequently Asked Questions (FAQs)
FAQ 1: What are the primary sources of reactive oxygen species production inside cells?
Understanding the origins of reactive oxygen species (ROS) is crucial for grasping how they impact health and aging. The main internal sources stem from normal cellular processes, particularly in energy production. Mitochondria, often called the cell’s powerhouses, are a major contributor because during the electron transport chain, a small percentage of oxygen gets partially reduced, leading to superoxide formation. This happens as electrons leak from complexes like I and III, creating these reactive molecules as byproducts.
Beyond mitochondria, enzymes play a significant role. For instance, NADPH oxidases deliberately generate ROS in immune cells to fight pathogens, while xanthine oxidase produces them during purine metabolism. Cytochrome P450 enzymes, involved in detoxifying substances, also release ROS as side effects. These sources ensure ROS are produced in controlled amounts for signaling but can overwhelm defenses if unchecked.
Other cellular components contribute too. Peroxisomes generate hydrogen peroxide during fatty acid breakdown, and endoplasmic reticulum stress can boost ROS output. In essence, while these sources are essential for life, their imbalance ties into oxidative stress and aging processes.
To organize this information clearly, here’s a detailed table outlining key internal sources of ROS, their mechanisms, and biological implications:
| Source of ROS | Mechanism of Production | Key ROS Produced | Biological Role | Potential Issues if Overproduced |
|---|---|---|---|---|
| Mitochondria | Electron leakage in the electron transport chain, especially at complexes I (NADH dehydrogenase) and III (ubiquinol-cytochrome c reductase) | Superoxide anion (O₂⁻), hydrogen peroxide (H₂O₂) | Energy production signaling, regulation of apoptosis | Mitochondrial DNA damage, accelerated aging, neurodegenerative diseases |
| NADPH oxidases (NOX enzymes) | Deliberate generation via electron transfer from NADPH to oxygen; seven isoforms exist with varying locations | Superoxide anion (O₂⁻) | Immune defense, cell signaling in vascular and inflammatory responses | Chronic inflammation, endothelial dysfunction in cardiovascular issues |
| Xanthine oxidase | Oxidation of xanthine to uric acid during purine catabolism | Superoxide anion (O₂⁻), hydrogen peroxide (H₂O₂) | Metabolic waste processing | Tissue damage in ischemia-reperfusion injuries, gout-related complications |
| Cytochrome P450 | Monooxygenase reactions in drug and toxin metabolism | Superoxide anion (O₂⁻), hydroxyl radical (HO•) | Detoxification of xenobiotics | Liver toxicity, increased cancer risk from DNA mutations |
| Peroxisomes | Beta-oxidation of fatty acids and other oxidative reactions | Hydrogen peroxide (H₂O₂) | Lipid metabolism, breakdown of very long-chain fatty acids | Peroxisomal disorders, oxidative overload in fatty liver disease |
| Endoplasmic reticulum | Protein folding stress (unfolded protein response) leading to oxidase activity | Hydrogen peroxide (H₂O₂), peroxynitrite (ONOO⁻) | Protein quality control | ER stress-linked diseases like diabetes and Alzheimer’s |
| Lipoxygenases and cyclooxygenases | Arachidonic acid metabolism in inflammation | Peroxyl radicals (ROO•) | Eicosanoid production for immune modulation | Exacerbated inflammatory conditions, arthritis progression |
| Nitric oxide synthases (uncoupled) | When cofactors are limited, produces superoxide instead of nitric oxide | Superoxide anion (O₂⁻) | Vascular tone regulation | Hypertension, impaired wound healing |
This table highlights how diverse these sources are, emphasizing their dual nature in maintaining cellular function while posing risks when dysregulated.
FAQ 2: How do reactive oxygen species influence the immune system?
Reactive oxygen species serve as key players in the body’s defense mechanisms, acting much like a double-edged sword in immunity. At moderate levels, they help immune cells communicate and respond effectively to threats. For example, during an infection, white blood cells like neutrophils and macrophages produce ROS through enzymes such as NADPH oxidase to create a burst that directly attacks bacteria and viruses. This oxidative burst is essential for killing pathogens by damaging their proteins, lipids, and DNA, ensuring the immune response is swift and targeted.
However, the story doesn’t end there. ROS also regulate more subtle aspects of immunity, including cell signaling and differentiation. They can activate transcription factors that guide immune cells to mature or migrate to infection sites. In adaptive immunity, recent studies suggest ROS might influence T-cell and B-cell functions, helping to fine-tune responses against specific invaders. But when ROS levels spike uncontrollably, they can turn against the body, promoting chronic inflammation or autoimmune reactions where healthy tissues are mistakenly attacked.
This balance is particularly relevant in aging, where declining antioxidant defenses allow ROS to accumulate, potentially weakening immune surveillance and increasing vulnerability to diseases. Emerging research from the past couple of years underscores how mitochondrial ROS maintain optimal immune cell concentrations, with either too much or too little disrupting physiological responses. In essence, managing ROS could be key to bolstering immunity without tipping into harmful territory.
The implications extend to various health conditions. In autoimmune disorders, excessive ROS might initiate responses by oxidizing self-molecules, making them appear foreign to the immune system. Conversely, in cancer, ROS can modulate tumor microenvironments, sometimes suppressing immune attacks. Scientists are exploring ways to harness this duality, like using therapies that precisely control ROS to enhance immune functions against pathogens or tumors while minimizing damage.
Overall, viewing ROS solely as harmful overlooks their vital role in protective immunity. As research evolves, it becomes clear that fostering a harmonious ROS environment could support healthier immune aging.
FAQ 3: Which foods rich in antioxidants can help combat oxidative stress?
Antioxidants are nature’s way of countering the buildup of reactive oxygen species, and incorporating them through diet is a practical approach to reducing oxidative stress. Foods packed with these compounds work by neutralizing free radicals, supporting cellular repair, and enhancing the body’s natural defenses. For instance, berries like blueberries and strawberries are standout choices due to their high flavonoid content, which not only scavenges ROS but also promotes anti-inflammatory effects.
To make this actionable, consider these food categories with specific examples and benefits:
- Berries and fruits: Blueberries, strawberries, raspberries, and goji berries top the list. They’re loaded with vitamin C and anthocyanins, which protect against lipid peroxidation and support heart health. Eating a cup daily can significantly boost plasma antioxidant levels.
- Nuts and seeds: Pecans, almonds, walnuts, and sunflower seeds provide vitamin E and selenium. These help regenerate other antioxidants like glutathione, crucial for detoxifying H₂O₂. A handful as a snack offers sustained protection against cellular damage.
- Vegetables: Dark leafy greens such as kale, spinach, and broccoli are rich in vitamins A, C, and K, plus compounds like sulforaphane. They enhance enzyme activity in the liver to break down toxins, reducing overall oxidative burden.
- Other sources: Dark chocolate (at least 70% cocoa) contains flavanols that improve endothelial function, while artichokes and beans offer fiber alongside antioxidants to aid gut health, indirectly lowering systemic stress.
Integrating these into meals doesn’t have to be complicated. A smoothie with berries and spinach, or a salad topped with nuts, can make a difference. Studies show diets like the Mediterranean, emphasizing these foods, correlate with lower oxidative markers and better longevity.
Beyond immediate effects, consistent intake supports long-term health by modulating gene expression related to antioxidant enzymes. Remember, variety is key to covering different types of antioxidants for comprehensive protection.
FAQ 4: What mechanisms do mitochondria use to generate reactive oxygen species?
Mitochondria are central to ROS production, primarily through their role in cellular respiration. The electron transport chain, a series of protein complexes in the inner mitochondrial membrane, transfers electrons from nutrients to oxygen, creating ATP. However, during this process, about 1-2% of electrons escape prematurely, reacting with oxygen to form superoxide anions. This leakage mainly occurs at complex I, where NADH is oxidized, and complex III, involving ubiquinone. These sites are hotspots because of their high electron flux, making mitochondria the body’s predominant ROS factory.
The generation isn’t random; it’s influenced by factors like metabolic rate and oxygen availability. Under high energy demand, reverse electron transport can amplify superoxide output at complex I, where electrons flow backward due to a buildup of reduced ubiquinone. Hydrogen peroxide forms next via superoxide dismutase in the matrix or intermembrane space, potentially diffusing out to affect other cell parts. Recent insights highlight how mitochondrial ROS also arise from other enzymes, like alpha-ketoglutarate dehydrogenase in the Krebs cycle, adding layers to this production.
This ROS output serves purposes beyond damage; it signals for mitochondrial biogenesis and quality control, ensuring damaged organelles are removed via mitophagy. Yet, in aging, inefficient chains increase leakage, creating a cycle of more ROS and further dysfunction. Understanding these mechanisms opens doors to interventions like targeted antioxidants that protect without disrupting signaling.
Advancements in research emphasize the compartmentalization: matrix-generated ROS stay local for signaling, while intermembrane ones might escape, influencing nuclear genes. This nuanced view shifts from seeing mitochondria solely as culprits to appreciating their regulatory role in health.
FAQ 5: How does physical exercise impact reactive oxygen species and muscle adaptation?
Exercise triggers a fascinating interplay with ROS, where short-term increases lead to long-term benefits for muscles. During workouts, ROS production rises from mitochondrial and non-mitochondrial sources, acting as signals for adaptation. This helps muscles grow stronger and more resilient, but overdoing it can cause fatigue.
Here’s a structured table detailing exercise types, their effects on ROS, and resulting adaptations:
| Exercise Type | ROS Production Trigger | Key ROS Involved | Muscle Adaptation Benefits | Potential Drawbacks if Excessive |
|---|---|---|---|---|
| Endurance (e.g., running, cycling) | Increased mitochondrial electron leakage and NADPH oxidase activation | Hydrogen peroxide (H₂O₂), superoxide (O₂⁻) | Enhanced mitochondrial biogenesis, improved endurance via PGC-1α activation | Muscle soreness, delayed recovery, oxidative damage to fibers |
| Resistance (e.g., weightlifting) | Muscle contraction-induced membrane depolarization | Peroxyl radicals (ROO•) | Protein synthesis boost, hypertrophy through mTOR pathway | Inflammation, temporary strength loss |
| High-Intensity Interval Training (HIIT) | Rapid energy demands causing reverse electron transport | Hydroxyl radical (HO•) in bursts | Faster metabolic adaptations, fat oxidation increase | Higher risk of cellular stress if not recovered properly |
| Aerobic Moderate (e.g., swimming) | Steady-state oxygen use elevating basal ROS | Nitric oxide (•NO) derivatives | Vascular improvements, better oxygen delivery to muscles | Minimal, but chronic without rest can accumulate stress |
| Yoga or Low-Impact | Mild metabolic shifts with breathing focus | Low-level H₂O₂ for signaling | Flexibility and recovery enhancement, reduced baseline inflammation | Rarely an issue, supports overall balance |
This illustrates how calibrated exercise uses ROS for positive changes, with antioxidants aiding recovery.
FAQ 6: What are the key biomarkers used to measure oxidative stress in the body?
Detecting oxidative stress involves tracking specific biomarkers that indicate ROS imbalance. These markers reflect damage to biomolecules or antioxidant depletion, providing insights into health risks.
Common categories include:
- Lipid peroxidation products: Malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) are widely used, measured in blood or urine via assays like TBARS. They signal membrane damage from ROS attacking fats.
- Protein oxidation markers: Carbonyl groups form on proteins, detectable through spectrophotometry or ELISA. High levels suggest impaired enzyme function.
- DNA/RNA damage indicators: 8-Hydroxydeoxyguanosine (8-OHdG) is a top choice, quantified in urine or serum, linking to mutation risks in aging.
- Antioxidant enzyme levels: Reduced superoxide dismutase (SOD) or glutathione peroxidase (GPx) activity, assessed enzymatically, shows defense weaknesses.
These biomarkers help diagnose conditions like diabetes or cardiovascular disease, guiding interventions.
Advanced methods like mass spectrometry offer precision, but simple blood tests suffice for routine checks.
FAQ 7: What external factors contribute to increased reactive oxygen species levels?
External influences can significantly ramp up ROS in the body, often compounding internal production and accelerating oxidative stress. Environmental pollutants like ozone and fine particulate matter from air pollution penetrate the lungs, triggering ROS generation in respiratory cells and spreading systemically. This exposure links to heightened inflammation and faster aging of tissues, as seen in urban dwellers facing chronic smog.
Radiation, including UV from sunlight and ionizing types from medical imaging, directly splits water molecules into hydroxyl radicals, damaging skin and DNA. Lifestyle choices play a big role too; smoking introduces thousands of free radicals per puff, overwhelming lungs and blood vessels. Even diet matters: high-sugar or processed foods promote advanced glycation end-products that spur ROS.
Medications and chemicals, such as certain chemotherapy drugs or pesticides, can induce ROS as side effects, necessitating antioxidant support. Recent findings emphasize how these factors interact with genetics, making some people more susceptible. Minimizing exposure through protective measures like sunscreen or clean air practices can curb this added burden.
FAQ 8: How have recent studies reshaped our understanding of ROS in the aging process?
The landscape of ROS research in aging has evolved dramatically in recent years, moving beyond the classic free radical theory. While earlier views pinned aging squarely on cumulative ROS damage, newer investigations reveal a more balanced picture where ROS drive adaptive responses. For instance, studies from 2024-2025 highlight how mitochondrial ROS at moderate levels promote hormesis, a stress response that enhances cellular resilience and potentially extends lifespan.
This shift emphasizes interconnections: ROS link with inflammation and immunity, forming a triad that influences age-related decline. Research shows improving mitochondrial function can delay aging by optimizing ROS output, not just reducing it. In models like extracellular vesicles, therapies target ROS to rejuvenate tissues.
Critically, excessive antioxidants might blunt beneficial ROS signals, explaining mixed supplement trial results. Instead, focus on lifestyle for natural balance. These insights suggest aging isn’t inevitable damage but a regulatable process.
FAQ 9: What is the link between reactive oxygen species and common age-related diseases?
ROS contribute to numerous diseases by promoting chronic oxidative stress, but their roles vary by condition. This connection underscores why managing ROS could prevent or mitigate these issues.
The following table explores key diseases, ROS involvement, and implications:
| Disease | ROS Contribution | Affected Systems | Prevention Insights | Research Notes |
|---|---|---|---|---|
| Cardiovascular Disease | Oxidation of LDL cholesterol leading to plaque; peroxynitrite impairs vessels | Heart, arteries | Antioxidant diets reduce risk | Linked to endothelial dysfunction |
| Neurodegenerative (e.g., Alzheimer’s, Parkinson’s) | Protein aggregation and neuronal death from mitochondrial ROS | Brain, nervous system | Exercise lowers brain ROS | Amyloid and tau oxidation central |
| Cancer | DNA mutations from hydroxyl radicals; tumor promotion via signaling | Various tissues | Balanced ROS for therapy response | Dual role: kills cancer cells too |
| Diabetes | Beta-cell damage and insulin resistance from high glucose-induced ROS | Pancreas, metabolism | Glycemic control key | Vascular complications amplified |
| Age-Related Macular Degeneration | Retinal cell harm via UV-triggered ROS | Eyes | Sunglasses, lutein-rich foods | Mitochondrial DNA mutations |
| Osteoarthritis | Cartilage breakdown from inflammatory ROS | Joints | Anti-inflammatory diets | Synovial fluid ROS elevation |
| Chronic Kidney Disease | Tubular damage and fibrosis from oxidative overload | Kidneys | Hydration, low-salt intake | Proteinuria worsens with ROS |
This overview shows ROS as a common thread in aging pathologies.
FAQ 10: How can everyday lifestyle changes help reduce the negative effects of ROS on aging?
Simple adjustments in daily habits can powerfully counteract ROS-driven aging by bolstering defenses and minimizing production. Start with diet: prioritizing whole foods over processed ones cuts down on inflammatory triggers that amplify ROS.
Effective strategies include:
- Regular physical activity: Moderate exercise like walking or yoga induces adaptive ROS for muscle and heart health, but avoid extremes to prevent overload.
- Stress management: Techniques such as meditation lower cortisol, which otherwise boosts ROS in cells.
- Sleep optimization: Aim for 7-9 hours nightly; poor sleep disrupts mitochondrial function, increasing ROS.
- Avoiding toxins: Limit alcohol and quit smoking to reduce external ROS sources.
These changes foster resilience, with studies showing they enhance antioxidant enzymes for better aging outcomes.
FAQ 11: What is the role of reactive oxygen species in cancer development and progression?
Reactive oxygen species play a complex and often contradictory role in the world of cancer, acting as both promoters of tumor growth and potential tools for destroying malignant cells. At the heart of this duality is their ability to influence cellular processes at different concentrations. In low to moderate levels, ROS can stimulate cancer cell proliferation by activating signaling pathways that encourage survival, migration, and even the formation of new blood vessels to feed tumors. This pro-tumorigenic effect stems from ROS causing DNA mutations, which accumulate over time and drive the initial stages of cancer development.
For example, chronic inflammation, a known risk factor for many cancers, often involves elevated ROS that damage genetic material and disrupt normal cell cycle controls.
As cancer progresses, ROS continue to shape the tumor microenvironment, a dynamic ecosystem where cancer cells interact with surrounding tissues. High ROS levels can enhance metastasis by breaking down extracellular matrices and promoting epithelial-mesenchymal transition, a process where cancer cells gain mobility. However, this isn’t always beneficial for the tumor; beyond a certain threshold, excessive ROS trigger oxidative stress that leads to cell death through mechanisms like apoptosis or ferroptosis. This vulnerability is why many chemotherapy drugs and radiation therapies aim to boost ROS in cancer cells, overwhelming their antioxidant defenses and selectively killing them while sparing healthier cells with better protective systems.
The implications for treatment are profound, with ongoing research exploring how to manipulate ROS for better outcomes. Some therapies focus on reducing ROS to prevent cancer initiation in high-risk individuals, while others amplify it to target established tumors. Challenges remain, such as drug resistance where cancer cells adapt by upregulating antioxidants like glutathione. Understanding these nuances could lead to personalized approaches, combining ROS-modulating agents with immunotherapies to tackle resistant cancers more effectively.
In summary, ROS aren’t simply villains in cancer; their role depends on context, timing, and levels. Balancing this act could unlock new strategies to halt progression and improve survival rates.
FAQ 12: Which supplements are effective in managing reactive oxygen species levels?
Navigating the world of supplements for ROS management requires understanding which ones have solid evidence behind them and how they work to maintain balance. Not all antioxidants are created equal, and while some show promise in reducing oxidative stress, others might not deliver in real-world scenarios or could even disrupt beneficial ROS signaling if overused.
To break it down clearly, here’s a comprehensive table of popular supplements, their mechanisms, evidence from studies, recommended dosages (general guidelines; consult a professional), and potential side effects:
| Supplement | Mechanism for Managing ROS | Key Evidence from Research | Typical Dosage | Potential Side Effects |
|---|---|---|---|---|
| Vitamin C (Ascorbic Acid) | Acts as a water-soluble antioxidant, regenerating vitamin E and neutralizing free radicals like hydroxyl radicals | Studies show it reduces markers of oxidative stress in smokers and those with chronic diseases; effective in combination with vitamin E | 500-2000 mg daily, split doses | Gastrointestinal upset at high doses, kidney stones in susceptible individuals |
| Vitamin E (Tocopherol) | Lipid-soluble, protects cell membranes from peroxidation by breaking radical chains | Proven to decrease lipid peroxidation in athletes and elderly; mixed results in large trials for heart disease prevention | 15-400 IU daily | Increased bleeding risk with blood thinners, possible prostate cancer link in high doses |
| Coenzyme Q10 (Ubiquinone) | Supports mitochondrial function, acts as an antioxidant in electron transport chain | Reduces ROS in heart failure patients and improves energy in aging; beneficial for statin users | 100-300 mg daily | Mild insomnia or nausea, rare allergic reactions |
| Alpha-Lipoic Acid | Regenerates other antioxidants like glutathione, chelates metals to prevent Fenton reactions | Effective in diabetic neuropathy by lowering ROS; animal studies show lifespan extension | 300-600 mg daily | Skin rashes, low blood sugar in diabetics |
| Resveratrol | Activates sirtuins, boosts antioxidant enzymes like SOD and catalase | Lowers oxidative stress in metabolic syndrome; inconsistent in human trials for aging | 150-500 mg daily | Digestive issues, interactions with blood thinners |
| Quercetin | Flavonoid that scavenges ROS and inhibits inflammatory pathways | Reduces exercise-induced oxidative damage; potential in allergy relief via ROS modulation | 500-1000 mg daily | Headaches, tingling; avoid with certain antibiotics |
| Selenium | Cofactor for glutathione peroxidase, enhances ROS detoxification | Linked to lower cancer risk in deficient populations; boosts GPx activity | 55-200 mcg daily | Hair loss or nail changes at excess levels |
| Melatonin | Regulates sleep and directly scavenges ROS, especially in mitochondria | Protects against age-related oxidative damage; useful in jet lag with antioxidant bonus | 0.5-5 mg nightly | Drowsiness, vivid dreams |
| N-Acetyl Cysteine (NAC) | Precursor to glutathione, replenishes cellular antioxidants | Effective in acetaminophen overdose and respiratory conditions by reducing ROS | 600-1800 mg daily | Nausea, rare anaphylaxis |
| Beta-Carotene | Provitamin A, quenches singlet oxygen in lipid phases | Mixed; beneficial in smokers for lung protection but increases risk in some | 3-15 mg daily | Skin yellowing, higher lung cancer risk in smokers |
| Curcumin (from Turmeric) | Inhibits NF-κB, boosts Nrf2 for antioxidant gene expression | Reduces inflammation and ROS in arthritis; poor bioavailability improved with piperine | 500-2000 mg daily with black pepper | Gallbladder issues in high doses |
| Glutathione | Master antioxidant, directly neutralizes H₂O₂ and peroxides | Oral forms show variable absorption; IV effective for liver detox | 250-500 mg daily | Rare bloating; not well-absorbed orally |
This table draws from various studies emphasizing targeted use rather than blanket supplementation. Always pair with a balanced diet for synergy.
FAQ 13: How do reactive oxygen species contribute to skin aging and wrinkles?
Reactive oxygen species are key players in the visible signs of skin aging, from fine lines to deeper wrinkles, by accelerating the breakdown of essential skin structures. When ROS levels rise due to factors like sun exposure or pollution, they attack collagen and elastin fibers, the proteins that keep skin firm and elastic. This degradation leads to sagging and wrinkling over time, as the skin loses its ability to bounce back.
To understand this better, consider these aspects of ROS impact on skin:
- UV radiation as a major trigger: Sunlight generates ROS in skin cells, causing photoaging that’s distinct from natural chronological aging. This results in uneven pigmentation and rough texture.
- Inflammation linkage: ROS promote chronic low-level inflammation, or inflammaging, which further damages dermal layers and exacerbates wrinkle formation.
- Antioxidant depletion: As we age, natural defenses like vitamin C drop, allowing ROS to dominate and speed up matrix metalloproteinase activity, enzymes that chew up collagen.
- Lifestyle influences: Smoking and poor diet amplify ROS, while protective habits like sunscreen use can mitigate effects.
Incorporating antioxidants topically or orally might help, but prevention through sun protection remains the gold standard for youthful skin.
FAQ 14: Are there gender differences in ROS production and aging?
Gender plays a subtle yet significant role in how reactive oxygen species are produced and managed during aging, influenced by hormones, genetics, and lifestyle. In general, males tend to exhibit higher baseline ROS production, partly due to greater mitochondrial activity and metabolic rates, which can lead to more oxidative damage accumulation over time. This might contribute to why men often experience certain age-related conditions earlier, like cardiovascular issues, though women catch up post-menopause when estrogen’s protective effects wane.
Estrogen, a key hormone in females, acts as an antioxidant by upregulating enzymes like superoxide dismutase, helping to keep ROS in check during reproductive years. This hormonal shield may explain why pre-menopausal women show lower oxidative stress markers compared to men of the same age. However, after menopause, declining estrogen levels shift the balance, increasing ROS-related risks such as bone loss and skin aging. Studies in animal models, like mice, reinforce this, showing female longevity advantages tied to better ROS homeostasis.
Genetics also factor in, with sex-specific gene expressions affecting antioxidant defenses. For instance, females often have more efficient repair mechanisms for oxidative damage, potentially extending healthspan. Yet, these differences aren’t absolute; environmental factors like diet and exercise can narrow the gap. Recognizing these variations could guide tailored anti-aging strategies, such as hormone therapies or targeted supplements.
Overall, while both genders face ROS-driven aging, the interplay of biology and hormones creates distinct paths, highlighting the need for gender-specific research in longevity.
FAQ 15: What is the connection between reactive oxygen species and mental health disorders?
The link between reactive oxygen species and mental health is emerging as a critical area, with oxidative stress implicated in disorders like depression, anxiety, and schizophrenia. ROS can disrupt brain chemistry by damaging neurons, altering neurotransmitter levels, and fueling inflammation that affects mood and cognition.
Here’s a detailed table outlining major mental health disorders, ROS involvement, underlying mechanisms, and potential interventions:
| Disorder | ROS Involvement | Key Mechanisms | Symptoms Linked to ROS | Potential Interventions |
|---|---|---|---|---|
| Depression | Elevated ROS from mitochondrial dysfunction | Oxidizes serotonin pathways, activates NLRP3 inflammasome | Low mood, fatigue, cognitive fog | Antioxidants like NAC, omega-3s; exercise to boost BDNF |
| Anxiety | NOX-derived ROS in amygdala | Heightens fear responses via GABA imbalance | Panic attacks, restlessness | Mindfulness, vitamin E supplementation |
| Schizophrenia | Increased ROS in dopamine neurons | Dopamine oxidation leads to hallucinations | Psychosis, disorganized thinking | Antipsychotics with antioxidant properties, coenzyme Q10 |
| Bipolar Disorder | Cyclic ROS spikes during mania | Mitochondrial mutations amplify oxidative bursts | Mood swings, impulsivity | Lithium (ROS modulator), melatonin for sleep |
| PTSD | Trauma-induced ROS in hippocampus | Impairs memory consolidation, neuroinflammation | Flashbacks, hypervigilance | Therapy combined with resveratrol |
| ADHD | ROS affecting prefrontal cortex | Dopamine transporter oxidation | Inattention, hyperactivity | Stimulants, flavonoid-rich diets |
| Alzheimer’s (as comorbidity) | Beta-amyloid triggers ROS | Tau protein oxidation | Memory loss | Curcumin, early antioxidant intervention |
This connection suggests ROS as a therapeutic target for mental health.
FAQ 16: How are reactive oxygen species levels measured in laboratory settings?
Measuring ROS in labs is essential for research on aging and disease, but it’s tricky due to their short lifespan and reactivity. Common methods rely on probes that react with ROS to produce detectable signals, allowing scientists to quantify levels in cells, tissues, or bodily fluids.
Key techniques include:
- Fluorogenic probes: Dyes like DCFH-DA fluoresce when oxidized by ROS, used in microscopy or flow cytometry for live-cell imaging.
- Spectrophotometry: Assays like TBARS measure lipid peroxidation products as indirect ROS indicators.
- Chemiluminescence: Probes emit light upon ROS reaction, sensitive for low levels.
- Electron spin resonance: Detects unpaired electrons in radicals for direct measurement.
- Mass spectrometry: Analyzes oxidized biomolecules for precise quantification.
These methods help track ROS dynamics, aiding drug development.
FAQ 17: What is the evolutionary significance of reactive oxygen species in living organisms?
From an evolutionary standpoint, reactive oxygen species have been pivotal since the dawn of aerobic life, serving as both challenges and catalysts for adaptation. Early organisms faced a toxic oxygen rise during the Great Oxidation Event, but those that harnessed ROS for signaling survived and thrived. This shift turned ROS from mere byproducts into essential regulators, enabling complex multicellular life by driving processes like apoptosis, which prunes unnecessary cells.
In evolutionary terms, ROS influenced key transitions, such as the development of antioxidant systems that balanced their destructive potential with beneficial roles in immunity and metabolism. Species with robust ROS management, like long-lived turtles, demonstrate how fine-tuning these molecules extends lifespan. Moreover, ROS may have spurred genetic diversity through mutations, accelerating evolution under stress.
This significance extends to modern organisms, where ROS mediate trade-offs between reproduction and longevity, as seen in theories linking metabolic rate to aging. Ultimately, ROS embody evolution’s ingenuity, transforming a threat into a tool for resilience.
FAQ 18: How does ROS function differ between plants and animals?
While reactive oxygen species serve similar roles in signaling and stress response across kingdoms, differences arise from unique cellular structures and environments. Plants, with chloroplasts and rigid cell walls, use ROS extensively in photosynthesis and defense against herbivores, whereas animals focus more on mitochondrial ROS for energy and immunity.
This table compares key functions, sources, and regulations:
| Aspect | In Plants | In Animals | Key Differences | Biological Implications |
|---|---|---|---|---|
| Primary Sources | Chloroplasts (photosystem I/II), peroxisomes, apoplast | Mitochondria, NADPH oxidases, peroxisomes | Plants have light-dependent sources; animals rely on metabolic | Plants handle diurnal ROS fluctuations; animals constant basal |
| Signaling Roles | Regulates stomatal closure, root growth, pathogen response | Controls cell proliferation, apoptosis, vascular tone | Plants use for abiotic stress; animals for neural signaling | Plants adapt to environment; animals to internal cues |
| Antioxidant Systems | Ascorbate-glutathione cycle prominent, flavonoids | SOD, catalase, glutathione dominant | Plants emphasize non-enzymatic; animals enzymatic | Plants resist UV; animals combat inflammation |
| Stress Response | ROS bursts for hypersensitive response killing infected cells | Phagocyte ROS for pathogen killing | Plants sacrifice tissue; animals target invaders | Plants contain spread; animals systemic immunity |
| Developmental Functions | Influences pollen tube growth, seed germination | Affects embryogenesis, wound healing | Plants link to hormones like auxin; animals to growth factors | Plants environmental integration; animals hormonal |
| Pathological Effects | Excess causes chlorosis, necrosis | Leads to neurodegeneration, cancer | Plants visible leaf damage; animals internal | Plants yield loss; animals chronic diseases |
These variances highlight evolutionary adaptations to lifestyles.
FAQ 19: What are emerging research directions in ROS and aging?
As we delve deeper into ROS and aging, new avenues are opening up, driven by advanced technologies and interdisciplinary approaches. One promising direction is mitochondrial therapies, like transplantation, to restore energy and reduce ROS leaks in aged cells.
Exciting areas include:
- Senolytics targeting ROS-driven senescence: Drugs clearing senescent cells to alleviate inflammaging.
- Nanomedicine for precise ROS modulation: Nanoparticles delivering antioxidants directly to mitochondria.
- Epigenetic interventions: Exploring how ROS alter gene expression for reversible aging markers.
- Microbiome links: Gut bacteria influencing systemic ROS and longevity.
- AI-driven modeling: Predicting ROS dynamics for personalized anti-aging.
These frontiers aim to extend healthspan by harnessing ROS wisely.
FAQ 20: What are the Common myths about reactive oxygen species and antioxidants debunked?
Many misconceptions surround reactive oxygen species (ROS) and antioxidants, often fueled by oversimplified narratives in health media. These myths can lead to unrealistic expectations or misguided health choices, so let’s set the record straight by addressing some of the most common misunderstandings. By exploring the science behind ROS and antioxidants, we can clarify their roles in aging and health, helping you make informed decisions without falling for hype.
One prevalent myth is that all ROS are inherently harmful and should be eliminated at all costs. This view paints ROS as villains, but they’re actually essential for life. At low levels, ROS like hydrogen peroxide act as signaling molecules, helping cells adapt to stress, fight infections, and regulate growth. For example, during exercise, a controlled increase in ROS triggers muscle strengthening. Completely wiping out ROS would disrupt these vital processes, potentially harming immunity or wound healing. The truth lies in balance—ROS are beneficial when managed by the body’s antioxidant systems but damaging when they overwhelm defenses, leading to oxidative stress.
Another widespread belief is that taking high doses of antioxidant supplements will prevent or reverse aging and disease. This idea stems from the free radical theory of aging, which links ROS damage to growing older. However, large-scale studies have shown mixed results. For instance, excessive vitamin E or beta-carotene supplements in smokers increased lung cancer risk rather than reducing it. Overloading on antioxidants can also suppress beneficial ROS signaling, like the adaptations that make exercise effective. A balanced diet rich in fruits, vegetables, and nuts often provides enough antioxidants without these risks, supporting the body’s natural defenses more effectively than mega-doses.
People also often assume that all ROS are the same, with identical effects on the body. In reality, ROS are a diverse group—superoxide, hydroxyl radicals, and peroxynitrite each have unique reactivities and roles. For example, superoxide can initiate signaling, while hydroxyl radicals are highly destructive and lack signaling functions. Treating them as a single entity oversimplifies their impact, leading to confusion about how to manage them. Understanding their differences helps explain why specific antioxidants target certain ROS more effectively.
Here are additional myths about ROS and antioxidants, debunked for clarity:
- Myth: Antioxidants can completely stop aging. While antioxidants like vitamin C or glutathione reduce oxidative damage, aging involves multiple factors—genetics, inflammation, and telomere shortening—beyond ROS alone. Studies show antioxidants can slow some aging markers, like skin wrinkles, but they don’t halt the process entirely. Lifestyle factors, such as sleep and stress management, are equally crucial.
- Myth: More antioxidants always mean better health. Flooding the body with supplements can disrupt redox balance, impairing ROS-dependent processes like immune responses. Research suggests moderate, dietary antioxidants, like those in berries, work best by complementing the body’s enzymes like superoxide dismutase.
- Myth: All free radicals cause cancer. While ROS can damage DNA and contribute to cancer initiation, they also play a role in killing cancer cells during treatments like chemotherapy, which leverages high ROS to target tumors. The context—amount and location—determines their effect.
- Myth: Antioxidants cure all diseases linked to oxidative stress. Conditions like Alzheimer’s or diabetes involve ROS, but antioxidants alone can’t cure them. For example, trials with vitamin E for heart disease prevention showed limited benefits, highlighting the need for holistic approaches including diet and exercise.
Finally, there’s a misconception that ROS only comes from internal sources like metabolism. External factors like UV radiation, pollution, or smoking significantly boost ROS levels, accelerating damage. This underscores the importance of protective measures, like sunscreen or quitting smoking, to complement internal antioxidant defenses.
By debunking these myths, we see that ROS and antioxidants are about balance, not extremes. Embracing a nuanced approach—through diet, lifestyle, and informed supplement use—helps harness their benefits while minimizing harm for healthier aging.
This understanding aligns with recent research emphasizing ROS as dual-natured molecules, not just agents of destruction. Focusing on natural sources of antioxidants and avoiding over-supplementation supports long-term health without disrupting the delicate dance of ROS in our cells.
Acknowledgement
The Examsmeta.com website expresses its sincere gratitude to the numerous reputable sources that provided invaluable insights and data for the article “Reactive Oxygen Species (ROS): Dual Roles in Aging, Health, and Disease.” Their comprehensive research and accessible information greatly enriched our understanding of reactive oxygen species (ROS), their biochemical mechanisms, and their implications in aging and disease.
Specifically, acknowledges the following websites for their contributions:
- National Institutes of Health (nih.gov) for detailed studies on oxidative stress and its role in cellular processes and age-related diseases.
- PubMed (pubmed.ncbi.nlm.nih.gov) for providing access to a vast repository of peer-reviewed articles on ROS signaling and antioxidant defenses.
- Nature (nature.com) for cutting-edge research on mitochondrial ROS production and its evolutionary significance.
- ScienceDirect (sciencedirect.com) for in-depth reviews on ROS in cancer, immunity, and neurodegeneration.
- American Physiological Society (physiology.org) for insights into the physiological impacts of ROS on muscle adaptation and cardiovascular health.

