Redox signaling stands as a fascinating and essential process in our cells, acting like a sophisticated communication network that helps regulate everything from daily functions to responses in tough situations. At its core, it involves reactive oxygen species, often called ROS, and other redox-active molecules that make precise, reversible tweaks to proteins. These changes then influence various cellular pathways and even how genes are expressed. While a balanced level of these reactive species supports normal body functions in what we know as redox biology, too much can tip the scale toward harmful oxidative stress.
Think of it as a delicate dance where molecules like hydrogen peroxide, or H₂O₂, and superoxide, or O₂⁻, play starring roles by oxidizing specific cysteine residues in proteins, sparking a wide array of cellular reactions. This process is not random chaos but a controlled system that keeps our bodies running smoothly.
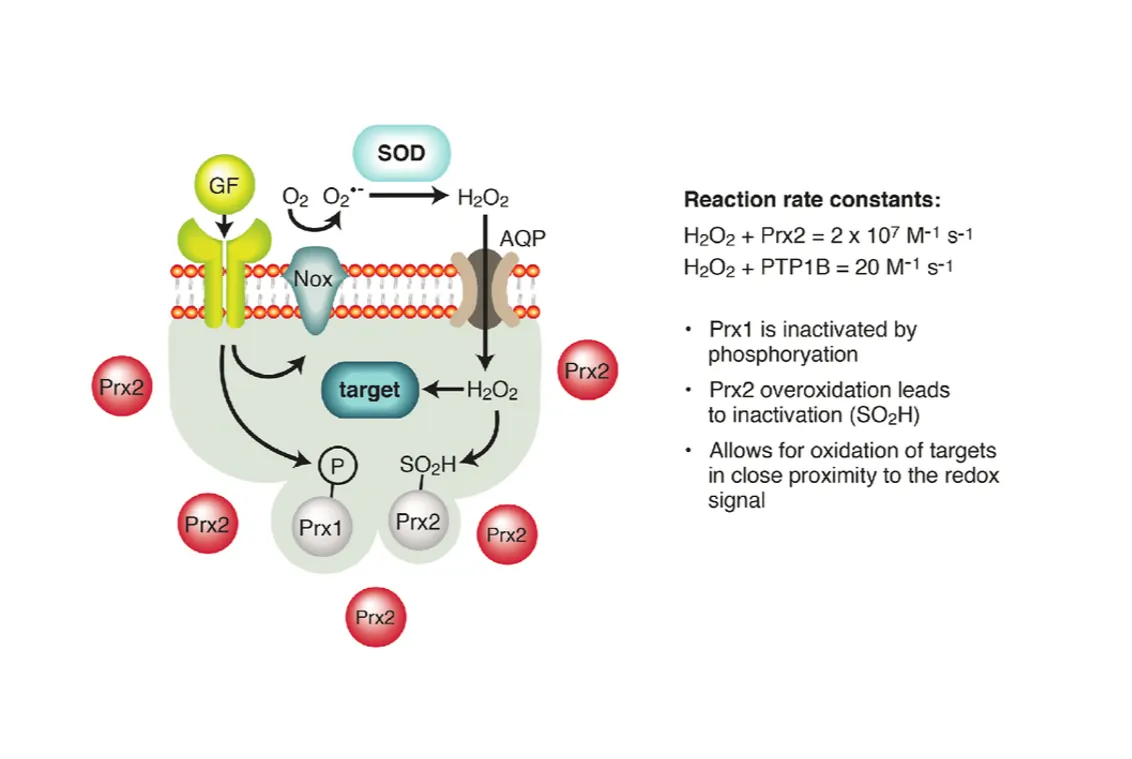
Table of Contents
In everyday life, redox signaling helps cells adapt to their environment, much like how a thermostat adjusts room temperature. For instance, when cells sense a mild increase in ROS, it can trigger protective mechanisms that boost antioxidant defenses or repair damaged parts. However, if the levels spike uncontrollably, it leads to damage in DNA, lipids, and proteins, contributing to various health issues. Researchers have delved deep into this, uncovering how this signaling bridges normal physiology and pathology, offering clues for new treatments. As we explore further, you’ll see how this seemingly simple electron transfer holds profound implications for human health.
What Exactly is Redox Signaling?
Redox signaling can be thought of as the cell’s way of using oxidation and reduction reactions to send messages. It revolves around electrophiles, which are molecules that attract electrons, interacting with nucleophiles, electron-donating parts like the thiol groups in cysteine residues of proteins. This interaction isn’t haphazard; it’s specific and often reversible, thanks to the cell’s built-in antioxidant systems that can undo the changes when needed.
One key aspect is the formation of covalent bonds or the transfer of electrons, leading to modifications that alter protein function. For example, a protein kinase might get activated, setting off a chain reaction that affects gene expression or cell growth. Unlike general oxidative damage, which is destructive and non-specific, redox signaling is precise, ensuring that only targeted proteins respond. This precision comes from factors like the location within the cell and the speed of reactions, allowing signals to be transmitted effectively without overwhelming the system.
Consider a real-world analogy: it’s like sending a text message versus shouting in a crowded room. The text is direct and reaches the intended recipient, while shouting might cause confusion. In cells, enzymes such as glutathione peroxidases and peroxiredoxins act as moderators, removing excess reactive species to keep the signaling clear and controlled.
How Redox Signaling Works in Detail
The mechanics of redox signaling start with the production of reactive species, which then interact with proteins to relay information. Reactive oxygen species (ROS) like superoxide and hydrogen peroxide are generated from sources such as mitochondria during energy production or enzymes like NADPH oxidase in response to stimuli. These species act as second messengers, similar to how hormones work but on a molecular scale.
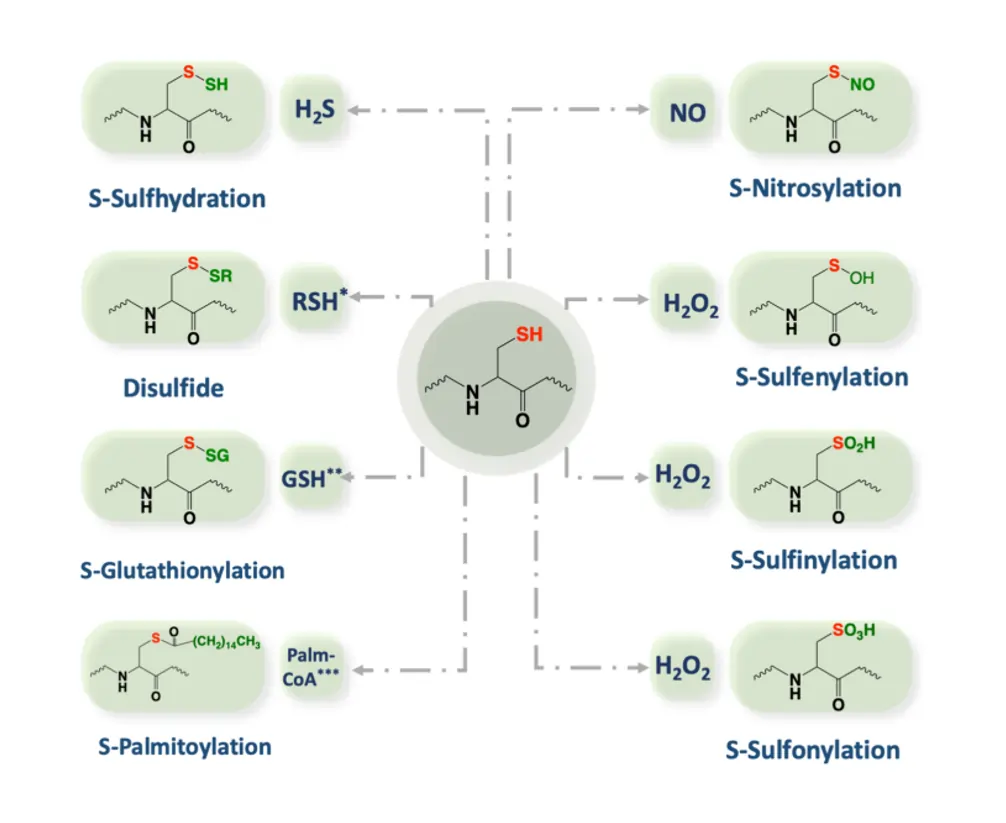
A crucial step is the oxidation of cysteine residues, which can form sulfenic acids, disulfides, or even attach glutathione in a process called glutathionylation. These modifications can switch proteins on or off, influencing pathways for cell survival, growth, or death. For reversal, reducing agents like NADPH or thioredoxin step in, restoring the proteins to their original state and ending the signal.
Types of reactions include oxidation, where electrons are removed, and addition, where bonds form covalently. In signaling, two-electron oxidations are common, avoiding the radical damage from one-electron processes. Specificity is key; proteins with reactive cysteines, often in low-pKa environments, are prime targets, ensuring the signal hits the right spot.
Take the example of hydrogen peroxide signaling: it can oxidize a sensor protein, leading to disulfide bond formation that activates a transcription factor, ultimately turning on genes for antioxidant production. This feedback loop maintains balance, preventing escalation to stress.
Key Molecules Involved in Redox Signaling
Several molecules drive this process, each with unique roles. Reactive oxygen species (ROS) top the list, with superoxide quickly converting to hydrogen peroxide, a more stable messenger that diffuses across cells. Hydrogen peroxide is particularly important because it selectively oxidizes cysteines without causing widespread damage.
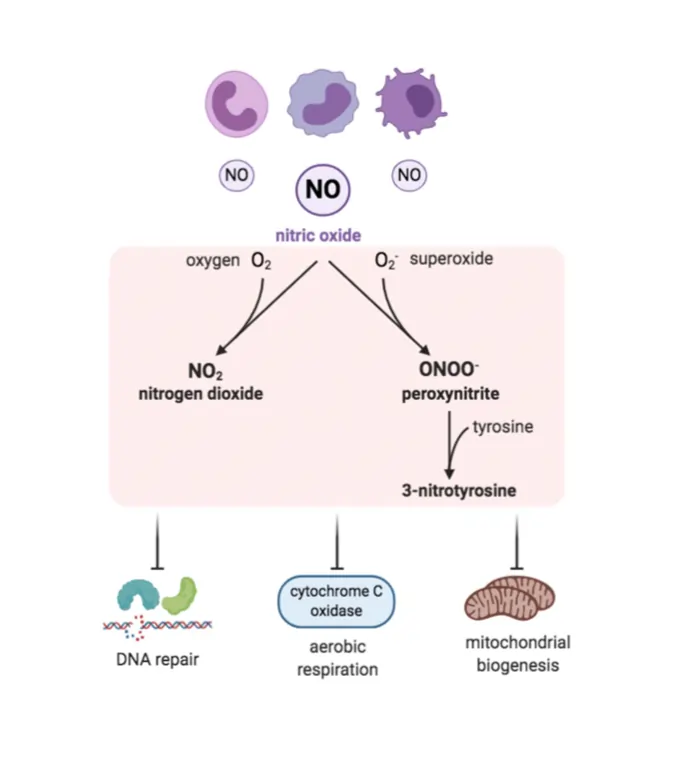
Reactive nitrogen species (RNS) like nitric oxide and peroxynitrite add another layer, often interacting with ROS to form potent signals. For instance, nitric oxide can S-nitrosylated proteins, modifying their activity in ways that regulate blood flow or immune responses.
Redox-active metals such as iron and copper facilitate electron transfers, acting as catalysts in reactions. Iron, in forms like Fe²⁺ and Fe³⁺, is vital in enzymes but can generate harmful radicals if not controlled.
Reducing equivalents, including NADPH, glutathione, and various thiols, provide the counterbalance, donating electrons to reverse oxidations. Glutathione, a tripeptide, is abundant in cells and conjugates with electrophiles to detoxify them.
Other players include lipid peroxidation products like 4-hydroxy-2-nonenal, which act as electrophiles in signaling, and enzymes like superoxide dismutase that convert superoxide to hydrogen peroxide, fine-tuning the reactive environment.
| Molecule Type | Examples | Primary Role | Physiological Impact |
|---|---|---|---|
| Reactive Oxygen Species (ROS) | Superoxide (O₂⁻), Hydrogen Peroxide (H₂O₂), Hydroxyl Radical (HO•) | Act as second messengers in signaling | Regulate cell proliferation, differentiation, and apoptosis; low levels promote health, high levels cause damage |
| Reactive Nitrogen Species (RNS) | Nitric Oxide (NO), Peroxynitrite (ONOO⁻) | Modify proteins via S-nitrosylation | Influence vasodilation, immune function, and inflammation control |
| Redox-Active Metals | Iron (Fe²⁺/Fe³⁺), Copper (Cu⁺/Cu²⁺) | Catalyze electron transfers | Essential for enzyme function but can amplify ROS if dysregulated |
| Reducing Equivalents | NADPH, Glutathione (GSH), Thioredoxin (Trx) | Reverse oxidative modifications | Maintain redox balance, support antioxidant defenses |
| Electrophiles from Lipids | 4-Hydroxy-2-nonenal (HNE), Isoprostanes | Covalent modification of proteins | Trigger stress responses, linked to inflammation and aging |
| Antioxidant Enzymes | Superoxide Dismutase (SOD), Catalase, Glutathione Peroxidase (GPx) | Neutralize excess ROS/RNS | Prevent oxidative stress, regulate signal duration |
This table highlights how these molecules interconnect, forming a robust network for cellular communication.
Redox Biology Versus Oxidative Stress
Redox biology refers to the beneficial side where controlled ROS levels orchestrate normal functions. A slight uptick in ROS might activate pathways for cell repair or adaptation to exercise, enhancing overall fitness. It’s like a gentle nudge that keeps systems alert and responsive.
In contrast, oxidative stress occurs when ROS overwhelm defenses, leading to irreversible harm. This imbalance is pathological, contributing to chronic conditions by damaging cellular components. For example, in aging, accumulated oxidative damage erodes mitochondrial function, accelerating decline.
The boundary between the two is fluid; what starts as signaling can escalate if antioxidants falter. Studies show that in healthy cells, redox biology supports processes like wound healing, where ROS guide immune cells to the site. But in diseased states, like diabetes, persistent oxidative stress inflames tissues, worsening insulin resistance.
The Importance of Redox Signaling in Cellular Regulation
Redox signaling is indispensable for maintaining cellular harmony. It modulates gene expression by activating transcription factors, ensuring cells respond aptly to stressors. In immune responses, for instance, ROS help activate NF-κB, a factor that ramps up inflammation when needed but must be dialed back to avoid chronic issues.
Beyond that, it influences metabolism, with redox states affecting nutrient sensing and energy production. Dysregulation here links to metabolic disorders, where faulty signaling disrupts glucose handling.
In development, redox gradients guide cell fate, from stem cell maintenance to differentiation. This underscores its role in regeneration, offering potential for therapies in tissue repair.
Overall, understanding this signaling opens doors to tackling diseases at their root, by restoring balance rather than just treating symptoms.
Major Signaling Pathways Influenced by Redox Processes
Redox signaling intersects with several key pathways, each regulating specific aspects of health.
The Keap1-Nrf2 pathway is a prime example, where oxidative stress modifies Keap1 cysteines, freeing Nrf2 to enter the nucleus and boost antioxidant genes. This pathway protects against environmental toxins and is crucial in longevity.
FOXO pathways respond to ROS by promoting stress resistance and autophagy, the cell’s cleanup process. In low-stress conditions, FOXOs are inhibited, but oxidation activates them for survival.
HIF pathways handle oxygen sensing; under low oxygen, HIF-1α stabilizes via redox modifications, upregulating genes for adaptation, like in wound healing or tumor survival.
NF-κB pathway gets activated by ROS to drive inflammation, essential for fighting infections but problematic in autoimmunity.
These pathways often crosstalk, amplifying or dampening signals for fine control.
| Pathway | Key Components | Redox Modification | Role in Health | Role in Disease |
|---|---|---|---|---|
| Keap1-Nrf2 | Keap1 (sensor), Nrf2 (transcription factor) | Cysteine alkylation or disulfide formation | Induces antioxidants, detoxifies xenobiotics | Overactivation in cancer promotes resistance; deficiency in neurodegeneration accelerates damage |
| FOXO | FOXO1/3/4, Transportin | Disulfide bonds with co-factors | Promotes longevity, autophagy, stress resistance | Dysregulation in diabetes leads to metabolic imbalance; in aging, reduced activity shortens lifespan |
| HIF | HIF-1α/2α, PHDs, pVHL | Proline hydroxylation, cysteine modifications | Adapts to hypoxia, supports angiogenesis | Hijacked in cancer for tumor growth; in cardiovascular disease, contributes to plaque instability |
| NF-κB | NF-κB subunits, IκB | ROS-induced phosphorylation | Regulates immune response, cell survival | Chronic activation in arthritis causes inflammation; in COPD, amplifies lung damage |
| ASK1 | ASK1 kinase, Trx, Prdx1 | Disulfide formation, Trx dissociation | Triggers apoptosis in stress | Overactive in heart failure promotes cell death; in hypertension, drives vascular remodeling |
This extensive table illustrates the breadth of redox influence on pathways, with examples drawn from physiological and pathological contexts.
Examples of Redox Signaling in Physiology
In normal body functions, redox signaling shines in various scenarios. During exercise, muscle cells produce ROS to signal for mitochondrial biogenesis, improving energy efficiency over time. This adaptation explains why regular workouts build endurance.
In the immune system, phagocytes use ROS bursts to kill pathogens, a controlled process that resolves once the threat passes. Nitric oxide signaling relaxes blood vessels, maintaining healthy blood pressure.
Developmental processes rely on it too; in embryogenesis, redox gradients direct cell migration and organ formation. In the brain, mild ROS levels support synaptic plasticity, aiding learning and memory.
Even in plants, though not human, similar principles apply, where ROS guide growth responses to light, offering comparative insights.
Redox Signaling’s Role in Diseases
When redox signaling goes awry, it fuels numerous diseases. In cancer, elevated ROS promote mutations and survival signals, but tumors often adapt by upregulating Nrf2 for protection against chemotherapy. This dual nature makes targeting tricky.
Neurodegenerative diseases like Alzheimer’s involve oxidative stress damaging neurons, with faulty autophagy failing to clear aggregates. Redox modifications impair proteins like ATG4B, exacerbating plaque buildup.
In cardiovascular diseases, ROS from sources like NOX contribute to atherosclerosis by oxidizing LDL, forming plaques. Hypertension sees redox signaling remodeling vessels, stiffening them.
Diabetes features insulin resistance from oxidative stress disrupting signaling pathways, while in COPD, cigarette smoke-induced ROS inflame lungs, reducing function.
Aging ties in as accumulated damage from mitoROS shortens telomeres and impairs repair, accelerating decline.
| Disease Category | Specific Examples | Redox Involvement | Key Mechanisms | Potential Biomarkers |
|---|---|---|---|---|
| Cancer | Prostate, Lung, Breast | Genomic instability, epigenetic changes | DNA damage from ROS, Nrf2 hijacking for resistance | 8-Hydroxyguanosine, Elevated H₂O₂ levels |
| Neurodegeneration | Alzheimer’s, Parkinson’s | Protein aggregation, impaired autophagy | Oxidation of ATG4B, mitochondrial dysfunction | F₂-Isoprostane, 4-Hydroxynonenal in brain tissue |
| Cardiovascular | Atherosclerosis, Hypertension, Heart Failure | Vascular remodeling, plaque formation | oxLDL promotion, ASK1 activation leading to apoptosis | Malondialdehyde, Oxidized LDL particles |
| Metabolic | Type 2 Diabetes, Obesity | Insulin resistance, metabolic reprogramming | Disruption of nutrient sensing, mTOR oxidation | Increased ROS in pancreatic islets, Reduced GSH |
| Respiratory | COPD, Idiopathic Pulmonary Fibrosis | Lung inflammation, fibrosis | Cigarette smoke ROS, NF-κB chronic activation | 8-Isoprostane in exhaled breath, Elevated NO levels |
| Aging-Related | Frailty, Sarcopenia | Mitochondrial decline, reduced repair | MitoROS accumulation, telomere shortening | Paraoxonase 1 activity decrease, HNE adducts |
This table provides a broad overview, emphasizing how redox dysregulation manifests across conditions.
Therapeutic Targets and Future Directions
Targeting redox signaling offers promise for treatments. Antioxidants like N-acetylcysteine replenish glutathione, showing benefits in Parkinson’s and diabetes trials. NRF2 activators, such as curcumin, are explored for Alzheimer’s and NAFLD.
Specific modulators include SOD mimics for inflammation and GPx mimetics like ebselen for stroke. Precision approaches focus on cysteine sites, like in KEAP1 for NRF2 stabilization.
Challenges remain, as broad antioxidants can disrupt beneficial signaling. Future research, aided by AI, aims for targeted drugs that correct imbalances without side effects.
In wrapping up, redox signaling emerges as a pivotal force, blending chemistry with biology to shape health. By harnessing its power, we might unlock new ways to combat diseases, fostering longer, healthier lives.
Frequently Asked Questions
FAQ 1: What Is Redox Signaling and Why Is It Important for Health?
Redox signaling represents a fundamental process in our bodies where cells use reactive molecules to communicate and adapt. Essentially, it involves the transfer of electrons between molecules, which triggers specific changes in proteins to regulate various functions. This isn’t just random chemistry; it’s a precise system that helps cells respond to their environment, much like how nerves transmit signals in the brain. For instance, when a cell encounters a mild stressor, such as during exercise, redox signaling can activate pathways that enhance energy production or repair mechanisms, keeping everything in balance.
At the heart of this process are reactive oxygen species, or ROS, which include compounds like hydrogen peroxide and superoxide. These act as messengers, oxidizing specific parts of proteins, particularly cysteine residues, to alter their shape and function. This modification can turn on or off genes, influence cell growth, or even decide if a cell should survive or undergo programmed death. What’s fascinating is that this signaling is reversible, thanks to antioxidants in the cell that can restore the proteins to their original state, preventing any long-term damage. Without this reversibility, the system would spiral into chaos, leading to issues like inflammation or tissue damage.
The importance of redox signaling extends far beyond basic cell function; it’s crucial for overall health. In healthy individuals, it supports immune responses by helping white blood cells target invaders, regulates blood flow through nitric oxide pathways, and even aids in wound healing by guiding cell migration. However, when disrupted, it contributes to chronic conditions. Research highlights how balanced redox signaling promotes longevity by maintaining cellular homeostasis, while imbalances accelerate aging. Understanding this can lead to better lifestyle choices, like incorporating antioxidant-rich foods to support these natural processes.
FAQ 2: How Does Redox Signaling Differ from Oxidative Stress?
Redox signaling and oxidative stress might seem similar since both involve reactive oxygen species, but they play very different roles in the body. Redox signaling is like a controlled conversation among cells, where low levels of ROS act as precise signals to modulate functions without causing harm. This process is essential for everyday activities, such as adapting to physical activity or responding to hormones. In contrast, oxidative stress occurs when ROS levels surge beyond the cell’s capacity to handle them, leading to widespread damage rather than targeted communication.
To break it down further, redox signaling relies on specificity: only certain proteins get modified, and these changes are temporary and beneficial. For example, a slight increase in hydrogen peroxide might activate a transcription factor to boost antioxidant production, creating a protective loop. Oxidative stress, however, is overwhelming and non-specific, attacking DNA, lipids, and proteins indiscriminately, which can result in mutations or cell death.
Key differences include:
- Level of ROS: Signaling uses low, controlled amounts, while stress involves high, unchecked levels that overwhelm defenses like superoxide dismutase or glutathione.
- Outcomes: Signaling promotes health through pathways like Nrf2 activation for detoxification, whereas stress contributes to diseases such as diabetes by impairing insulin signaling or accelerating atherosclerosis in heart conditions.
- Reversibility: In signaling, modifications are easily reversed; in stress, damage often becomes permanent, linking to chronic inflammation.
Recognizing these distinctions helps in appreciating how maintaining redox balance through diet and exercise can prevent the shift from helpful signaling to harmful stress.
FAQ 3: What Are the Main Molecules Involved in Redox Signaling?
| Molecule Category | Specific Examples | Function in Signaling | Impact on Health | Sources of Production |
|---|---|---|---|---|
| Reactive Oxygen Species (ROS) | Hydrogen Peroxide (H₂O₂), Superoxide (O₂⁻), Hydroxyl Radical (HO•) | Serve as primary messengers by oxidizing cysteine residues in proteins to trigger pathways like MAPK or NF-κB | At low levels, support immune function and cell repair; excess leads to DNA damage and aging | Mitochondria during respiration, NADPH oxidases in response to stimuli |
| Reactive Nitrogen Species (RNS) | Nitric Oxide (NO), Peroxynitrite (ONOO⁻) | Modify proteins through S-nitrosylation, influencing blood vessel relaxation and inflammation control | Essential for cardiovascular health and wound healing; dysregulation linked to hypertension and autoimmune issues | Endothelial cells via nitric oxide synthase, reaction of superoxide with NO |
| Redox-Active Metals | Iron (Fe²⁺/Fe³⁺), Copper (Cu⁺/Cu²⁺) | Catalyze electron transfers in enzymes, amplifying signals in pathways like Fenton reactions | Vital for hemoglobin function and enzyme activity; imbalance causes neurotoxicity in diseases like Parkinson’s | Dietary intake, stored in ferritin or ceruloplasmin |
| Reducing Agents | Glutathione (GSH), Thioredoxin (Trx), NADPH | Reverse oxidative modifications to terminate signals and restore protein function | Maintain cellular balance, prevent oxidative stress; depletion associated with liver diseases and diabetes | Synthesized in cells from amino acids, regenerated by glucose-6-phosphate dehydrogenase |
| Antioxidant Enzymes | Superoxide Dismutase (SOD), Catalase, Glutathione Peroxidase (GPx) | Neutralize excess ROS to fine-tune signaling duration and prevent escalation to stress | Protect against cancer and neurodegeneration; mutations increase susceptibility to environmental toxins | Expressed in response to Nrf2 activation, found in peroxisomes and mitochondria |
| Lipid-Derived Electrophiles | 4-Hydroxy-2-Nonenal (HNE), Isoprostanes | Covalently bind to proteins, activating stress responses via Keap1-Nrf2 pathway | Involved in inflammation resolution; chronic elevation promotes fibrosis in lungs and kidneys | Lipid peroxidation during oxidative bursts in immune cells |
This table outlines the core players, showing how they interconnect to orchestrate redox processes while highlighting their dual roles in health maintenance and disease risk.
FAQ 4: How Does Redox Signaling Regulate Cellular Pathways?
Redox signaling serves as a master regulator in cells, influencing everything from energy metabolism to gene expression. It starts with ROS generated in mitochondria or by enzymes like NADPH oxidase, which then target sensitive proteins. These oxidations can activate kinases, leading to cascades that adjust cellular behavior. For example, in response to low oxygen, redox changes stabilize HIF-1α, prompting genes for new blood vessel formation, which is vital in healing but can be hijacked in tumors.
Beyond that, this signaling integrates with other systems, such as hormonal responses. Insulin signaling, for instance, involves redox modifications that enhance glucose uptake, but chronic high ROS can desensitize these pathways, contributing to type 2 diabetes. In the immune system, redox signals help differentiate T-cells, ensuring a balanced response to infections without overreacting into autoimmunity.
Overall, the beauty of redox signaling lies in its adaptability. It allows cells to sense and respond to internal and external cues dynamically, promoting resilience. Disruptions, however, can lead to pathological states, underscoring the need for balanced lifestyles to support this intricate network.
FAQ 5: Which Signaling Pathways Are Most Affected by Redox Processes?
| Pathway Name | Core Components | Redox Modifications Involved | Physiological Roles | Disease Associations |
|---|---|---|---|---|
| Keap1-Nrf2 | Keap1 sensor, Nrf2 transcription factor | Cysteine oxidation or alkylation leading to Nrf2 release | Detoxification of toxins, antioxidant gene expression for cellular protection | Cancer resistance through overactivation; neurodegeneration from deficiency causing unchecked damage |
| FOXO | FOXO1/3/4 factors, associated transport proteins | Disulfide bond formation with co-regulators | Enhances longevity, promotes autophagy and stress resistance mechanisms | Metabolic disorders like diabetes from impaired activity; accelerated aging with reduced signaling |
| HIF | HIF-1α/2α subunits, prolyl hydroxylases (PHDs), von Hippel-Lindau protein | Proline hydroxylation and cysteine tweaks under hypoxia | Adaptation to low oxygen, angiogenesis for tissue repair | Tumor progression in cancers; unstable plaques in cardiovascular conditions |
| NF-κB | NF-κB subunits, IκB inhibitors | ROS-triggered phosphorylation and degradation of IκB | Immune regulation, promotion of cell survival during infections | Chronic inflammation in arthritis; lung tissue damage in chronic obstructive pulmonary disease |
| MAPK | ERK1/2, JNK, p38 kinases | Direct oxidation of cysteines or indirect via upstream activators | Cell proliferation, differentiation, and apoptosis control | Cancer growth from sustained activation; inflammatory responses in rheumatoid conditions |
| ASK1 | ASK1 kinase, Thioredoxin (Trx), Peroxiredoxin-1 (Prdx1) | Disulfide bonds causing Trx dissociation | Initiates apoptosis under severe stress | Cardiac cell death in heart failure; vascular changes in hypertension |
| PI3K/AKT | PI3K enzyme, AKT kinase | Redox-sensitive cysteine residues in AKT | Growth factor signaling, cell survival and metabolism | Insulin resistance in diabetes; tumor survival in various cancers |
These pathways demonstrate the extensive reach of redox signaling, with each responding uniquely to oxidative cues to maintain health or contribute to pathology when imbalanced.
FAQ 6: What Are Some Everyday Examples of Redox Signaling in the Body?
Redox signaling isn’t just a lab concept; it’s active in our daily lives, helping the body function smoothly. During physical activity, for instance, muscles generate ROS to signal for more mitochondria, improving stamina over time. This adaptation is why consistent exercise boosts energy levels and overall fitness.
Other examples abound:
- In immune defense, ROS bursts from neutrophils kill bacteria at infection sites, with signaling ensuring the response stops once the threat is gone, preventing unnecessary tissue harm.
- Blood pressure regulation involves nitric oxide, a redox molecule that relaxes vessels, maintaining healthy circulation and reducing heart strain.
- Brain function benefits from mild ROS supporting synaptic changes, which underpin learning and memory formation during activities like reading or problem-solving.
- Wound healing relies on redox gradients to attract repair cells, speeding recovery from cuts or injuries.
These instances show how redox signaling quietly supports vitality, emphasizing the value of habits like balanced nutrition to keep it optimal.
FAQ 7: How Is Redox Signaling Linked to Cancer Development?
Redox signaling plays a complex, dual role in cancer, acting both as a promoter and a potential suppressor depending on the context. In early stages, elevated ROS from sources like mitochondrial dysfunction can cause DNA mutations, driving cells toward uncontrolled growth. This oxidative environment modifies proteins in pathways such as PI3K/AKT, enhancing survival signals that allow cancer cells to evade death. Moreover, tumors often exploit redox signaling to adapt to harsh conditions, like low oxygen, by stabilizing HIF factors that promote new blood vessels for nutrient supply.
As cancer progresses, cells develop resistance through upregulated antioxidants, hijacking the Nrf2 pathway to shield themselves from chemotherapy-induced stress. This adaptation makes treatments less effective, highlighting why targeting specific redox components could improve outcomes. Interestingly, some therapies aim to overwhelm this balance, using pro-oxidants to push cancer cells into fatal oxidative stress while sparing healthy ones.
Research continues to unravel these mechanisms, suggesting that personalized approaches based on a tumor’s redox profile might revolutionize treatment, focusing on restoring normal signaling to halt progression.
FAQ 8: What Role Does Redox Signaling Play in Neurodegenerative Diseases?
In neurodegenerative diseases, redox signaling often shifts from protective to destructive, contributing to progressive neuron loss. Conditions like Alzheimer’s involve excessive ROS damaging brain proteins, leading to aggregates that impair function. Here, faulty redox modifications disrupt autophagy, the cell’s recycling system, allowing toxic buildups.
Parkinson’s similarly features redox imbalances in dopamine-producing cells, where iron-catalyzed reactions amplify oxidative damage, triggering cell death pathways like ASK1. This creates a vicious cycle, exacerbating symptoms like tremors.
Huntington’s disease sees mutant proteins sensitive to oxidation, altering signaling that normally supports neuron survival. Overall, these disorders underscore how dysregulated redox processes accelerate decline, but emerging therapies target restoration for potential slowdown.
Key aspects include:
- Mitochondrial ROS overload impairing energy production in affected neurons.
- Reduced antioxidant defenses, like lowered glutathione, heightening vulnerability.
- Inflammation driven by NF-κB activation, worsening tissue damage.
Addressing these through antioxidants shows promise in slowing progression.
FAQ 9: Are There Effective Therapeutic Targets in Redox Signaling for Diseases?
Yes, therapeutic targets in redox signaling hold great potential for treating various diseases by restoring balance. Antioxidants like N-acetylcysteine work by boosting glutathione levels, helping in conditions such as Parkinson’s where oxidative stress depletes defenses. Clinical trials have shown improvements in motor symptoms by modulating these pathways.
Other strategies include:
- Nrf2 activators, such as sulforaphane from broccoli, which enhance detoxification genes to combat neurodegeneration and cancer resistance.
- SOD mimics that neutralize superoxide, reducing inflammation in arthritis and protecting heart tissue post-infarction.
- Nitric oxide donors for cardiovascular issues, improving vessel function by fine-tuning RNS signaling.
- Targeted inhibitors of NADPH oxidases to curb excessive ROS in hypertension and diabetes, preventing vascular damage.
These approaches emphasize precision to avoid disrupting beneficial signaling, with ongoing research refining them for better efficacy.
FAQ 10: What Are the Latest Advances in Redox Signaling Research?
Recent advances in redox signaling research have unveiled exciting insights, particularly in how super sulfides—sulfur-linked molecules—govern new aspects of cellular communication. These species offer a fresh perspective beyond traditional ROS, influencing everything from energy metabolism to immune modulation, potentially reshaping treatments for inflammatory diseases.
In 2025, studies have highlighted tissue-specific models and inhibitors for proteins like ERO1-PDI, advancing our grasp of endoplasmic reticulum stress in conditions such as diabetes. This has led to pharmacologic tools that selectively target redox pathways, minimizing side effects.
Moreover, the interplay between redox and autophagy has gained traction, with findings showing how oxidation fine-tunes cell cleanup in cancer, opening doors for combined therapies. AI-driven analyses are accelerating discoveries, predicting redox profiles for personalized medicine.
Overall, these developments promise transformative interventions, from antioxidant enhancements to novel drugs that harness signaling for healthier aging and disease prevention.
FAQ 11: How Does Redox Signaling Contribute to Aging?
Redox signaling plays a pivotal role in the aging process, acting as both a regulator of cellular health and a potential driver of decline when imbalanced. As we age, the body’s ability to maintain redox homeostasis diminishes, leading to increased oxidative modifications that affect proteins, lipids, and DNA. This shift can activate pathways associated with senescence, where cells stop dividing but remain active, contributing to tissue dysfunction.
For example, mitochondrial dysfunction generates excess ROS, which disrupts signaling and accelerates telomere shortening, a hallmark of aging. Recent studies emphasize that compartmentalized ROS production allows for localized control, but in older individuals, this precision falters, resulting in widespread damage.
Furthermore, redox changes intersect with other aging factors like sleep and metabolism. Poor sleep disrupts circadian rhythms, altering redox metabolism and heightening vulnerability to age-related diseases such as dementia. The interplay between sleep deprivation and oxidative stress creates a cycle where reduced antioxidant capacity exacerbates neuronal damage, linking redox imbalance to cognitive decline. Emerging research suggests that interventions like dietary restriction can mitigate these effects by enhancing cysteine-mediated signaling, which supports protein stability and reduces age-associated redox stress.
In essence, while physiological redox signaling promotes longevity through adaptive responses, its dysregulation in aging leads to chronic inflammation and metabolic reprogramming. This understanding opens avenues for therapies that target redox pathways to extend healthspan, focusing on restoring balance rather than eliminating all reactive species.
FAQ 12: What Is the Connection Between Redox Signaling and Cardiovascular Diseases?
| Aspect | Mechanisms Involved | Key Redox Players | Health Implications | Therapeutic Insights |
|---|---|---|---|---|
| Vascular Function | ROS from NADPH oxidases oxidize proteins, leading to endothelial dysfunction and impaired nitric oxide signaling | Hydrogen peroxide, Superoxide, Nitric oxide | Contributes to hypertension by stiffening arteries and promoting plaque buildup | Antioxidants like vitamin C may restore endothelial health by scavenging excess ROS |
| Ischemia-Reperfusion Injury | Sudden ROS bursts during reperfusion damage cardiomyocytes via lipid peroxidation and protein modifications | Hydroxyl radicals, Peroxynitrite | Increases risk of heart attacks and post-infarction complications like arrhythmias | Preconditioning with mild exercise enhances adaptive redox signaling to protect against severe damage |
| Atherosclerosis | Oxidized LDL triggers inflammatory redox pathways, activating NF-κB for cytokine release | Lipid-derived electrophiles like 4-HNE | Accelerates plaque formation and vascular inflammation, leading to strokes | Statins reduce ROS production, indirectly modulating redox balance for plaque stabilization |
| Cardiac Remodeling | Chronic oxidative stress alters kinase signaling, promoting fibrosis and hypertrophy | Thioredoxin, Glutathione peroxidase | Results in heart failure through maladaptive changes in muscle structure | Targeting ASK1 pathways with inhibitors could prevent fibrosis by controlling apoptotic signals |
| Autophagy Interplay | Redox signals regulate autophagic flux; excess ROS inhibits it, leading to cellular debris accumulation | Mitochondrial ROS, Nrf2 activation | Worsens cardiomyopathy in diabetic hearts by impairing cleanup mechanisms | Exercise-induced ROS waves promote beneficial autophagy, improving cardiac resilience |
| Biomarkers | Elevated 3-nitrotyrosine and oxidized DNA bases indicate ongoing redox imbalance | Protein carbonyls, 8-OHdG | Serve as predictors for disease progression and response to treatments | Monitoring these helps tailor interventions like antioxidant supplements for at-risk individuals |
This table illustrates the multifaceted links, drawing from physiological and pathological contexts to highlight prevention strategies.
FAQ 13: How Does Redox Signaling Affect Diabetes and Metabolic Health?
Redox signaling influences diabetes by modulating insulin pathways and metabolic reprogramming, where controlled ROS levels support normal glucose handling but excess leads to resistance. In pancreatic beta cells, glucose stimulates mild ROS production for insulin secretion, yet chronic high levels cause oxidative modifications that impair function, contributing to type 2 diabetes onset.
Key effects include:
- Insulin Resistance: Elevated ROS disrupt AKT signaling, reducing glucose uptake in muscles and liver, exacerbated by obesity-linked inflammation.
- Beta Cell Dysfunction: Oxidative stress from hyperglycemia damages mitochondria, leading to apoptosis and reduced insulin output.
- Metabolic Syndrome: Redox imbalances promote fat accumulation and dyslipidemia, with circadian disruptions worsening the cycle.
- Complications: Vascular redox changes accelerate neuropathy and retinopathy through endothelial damage.
Balancing this through lifestyle can restore signaling for better metabolic control.
FAQ 14: What Role Does Redox Signaling Play in the Immune System?
Redox signaling is integral to immune function, orchestrating responses from activation to resolution. Innate immune cells like macrophages use ROS bursts to kill pathogens, with signaling ensuring targeted action without excessive inflammation. For adaptive immunity, redox modifications regulate T-cell differentiation, balancing pro- and anti-inflammatory states.
In detail, nuclear factor-κB activation by ROS drives cytokine production during infections, while antioxidant systems like thioredoxin prevent overreaction. Dysregulation links to autoimmunity, where persistent oxidative stress sustains chronic responses. Recent findings show redox-sensitive proteins in memory formation, enhancing future defenses.
Overall, this signaling maintains immune homeostasis, with implications for therapies in infections and cancer.
FAQ 15: Can Diet Influence Redox Signaling?
| Dietary Component | Examples | Impact on Redox Signaling | Health Benefits | Potential Risks |
|---|---|---|---|---|
| Antioxidants | Vitamin C, Vitamin E, Beta-carotene | Scavenge ROS to prevent overload, supporting reversible signaling | Reduces inflammation, aids in disease prevention like diabetes | Over-supplementation may disrupt beneficial ROS signals |
| Polyphenols | Curcumin, Resveratrol from turmeric and grapes | Activate Nrf2 for antioxidant gene expression | Enhances detoxification, promotes longevity | High doses could interact with medications |
| Omega-3 Fatty Acids | Fish oil, Flaxseeds | Modulate lipid peroxidation, reducing inflammatory ROS | Improves cardiovascular health, balances metabolic signaling | Excessive intake might affect blood clotting |
| Sulfur-Containing Foods | Garlic, Broccoli | Boost glutathione production for redox reversal | Supports immune function, detoxifies pollutants | May cause digestive issues in some |
| Micronutrients | Zinc, Selenium | Cofactors for enzymes like SOD, fine-tuning ROS levels | Prevents deficiency-related oxidative stress | Imbalances lead to toxicity |
| Whole Plant Foods | Berries, Leafy greens | Provide fiber and phytonutrients for microbiome-redox interplay | Maintains gut health, indirect redox support | Low variety diets miss broad protection |
This table shows how diet shapes signaling for optimal health.
FAQ 16: How Does Exercise Impact Redox Signaling?
Exercise profoundly affects redox signaling, inducing adaptive ROS production that strengthens cellular defenses. Moderate activity generates controlled ROS waves, activating pathways for mitochondrial biogenesis and antioxidant upregulation, enhancing endurance.
Notable impacts include:
- Muscle Adaptation: ROS trigger gene expression for repair and growth, improving performance.
- Cardiovascular Benefits: Enhances endothelial function via nitric oxide signaling.
- Anti-Aging Effects: Promotes autophagy, clearing damaged components.
- Disease Prevention: Reduces chronic stress in diabetes and heart conditions.
Regular exercise optimizes this for long-term health.
FAQ 17: What Environmental Factors Affect Redox Signaling?
Environmental factors like pollutants disrupt redox signaling, shifting balance toward stress. Air pollution, with particulate matter, induces ROS in lungs and bloodstream, activating inflammatory pathways and contributing to cardiovascular risks.
Heavy metals and pesticides generate free radicals, overwhelming antioxidants and leading to DNA damage. Noise pollution alters circadian redox rhythms, exacerbating metabolic issues.
These exposures highlight the need for protective measures to preserve signaling integrity.
FAQ 18: What Are Emerging Therapies Targeting Redox Signaling?
| Therapy Type | Examples | Mechanism | Target Diseases | Current Status |
|---|---|---|---|---|
| Nrf2 Activators | Sulforaphane, Bardoxolone | Enhance antioxidant genes via Keap1 modulation | Neurodegeneration, Cancer | Clinical trials for kidney disease |
| ROS Modulators | MitoQ, SS-31 | Target mitochondrial ROS to restore balance | Aging, Heart failure | Preclinical for senescence |
| Cysteine-Targeted | N-acetylcysteine derivatives | Support reversible protein modifications | Aging, Inflammation | Emerging for longevity interventions |
| Autophagy Enhancers | Rapamycin analogs | Link redox to cellular cleanup | Cancer, Diabetes | Approved for some uses, expanding |
| Wound Healing Agents | Redox-modulating hydrogels | Promote signaling for repair | Chronic wounds | In development for diabetes ulcers |
| Brain-Specific | Glutathione boosters | Address oxidative stress in neurons | Alzheimer’s, Parkinson’s | Early trials for cognitive decline |
This table outlines promising approaches as of 2025.
FAQ 19: What Are Common Myths About Redox Signaling and Oxidative Stress?
Many believe all ROS are harmful, but they serve essential signaling roles at low levels, only causing issues when excessive.
Myths include:
- Antioxidants Always Help: Overuse can hinder beneficial signals.
- Oxidative Stress Is Solely Aging’s Cause: It’s one factor among many.
- More Supplements Mean Better Protection: Balance is key, not quantity.
Dispelling these promotes informed health choices.
FAQ 20: What Are Ways to Measure Redox Status in the Body?
Measuring redox status involves biomarkers reflecting oxidative balance, crucial for assessing health risks. Common methods include assaying total antioxidant capacity in blood, which gauges defense against ROS.
Specific markers like glutathione ratios indicate cellular redox state, with lower reduced forms signaling stress. Advanced techniques measure protein carbonyls or 8-OHdG for damage extent.
These tools aid in monitoring interventions for personalized care.
Acknowledgement
The Examsmeta.com website sincerely expresses its gratitude to the following reputable sources for providing valuable insights and data that significantly enriched the article “Redox Signaling: Mechanisms, Health Impact, and Disease Connections.” Their comprehensive resources and up-to-date research were instrumental in ensuring the accuracy and depth of the content.
Specifically, acknowledges PubMed (pubmed.ncbi.nlm.nih.gov) for its extensive database of peer-reviewed scientific studies, Nature (www.nature.com) for its cutting-edge research articles, ScienceDirect (www.sciencedirect.com) for its in-depth publications on cellular biology, and NCBI (www.ncbi.nlm.nih.gov) for its accessible genetic and biochemical resources.
These platforms provided critical information on redox signaling mechanisms, pathways, and their implications in health and disease, enabling a well-rounded and authoritative exploration of the topic. Additionally, I extend thanks to the scientific community for their ongoing efforts to advance our understanding of redox biology, which made this article possible.

