Cell signaling is one of the most fascinating and essential processes in biology, allowing cells to communicate, respond to their environment, and coordinate complex activities. Whether it’s a single bacterium sensing its neighbors or a human neuron firing in response to a thought, cell signaling underpins nearly every aspect of life.
In this comprehensive article, we’ll dive deep into what cell signaling is, its stages, types, receptors, functions, and much more. We’ll explore how it works across different organisms, highlight real-world examples, and discuss recent insights into its role in health and disease. By the end, you’ll have a clear picture of why this intricate system is crucial for everything from growth to survival.
Table of Contents
Defining Cell Signaling
Cell signaling refers to the ways cells send, receive, and respond to messages from their surroundings or from within themselves. These messages help cells perform tasks like growing, dividing, or even dying when necessary. It’s like a sophisticated language that cells use to stay in sync with each other and adapt to changes.
Imagine a bustling city where traffic lights, road signs, and pedestrian signals keep everything moving smoothly. Similarly, cell signaling ensures that biological processes run without chaos. Without it, multicellular organisms couldn’t develop properly, and even single-celled life forms would struggle to survive in dynamic environments.
Cell signaling isn’t just about external communication; it also includes internal dialogues within a single cell. For instance, when a cell detects damage to its DNA, internal signals might trigger repair mechanisms or, if the damage is too severe, initiate programmed cell death to prevent issues like cancer.
What is Cell Signaling?
Expanding on the definition, cell signaling is the mechanism through which cells interact with one another or respond to internal cues to carry out various physiological tasks. Also known as cellular signaling, this process is indispensable for the proper functioning of both prokaryotic (like bacteria) and eukaryotic cells (found in plants, animals, and humans).
Signals can be physical, such as changes in temperature, pressure, or electrical currents, or chemical, including hormones like insulin, ions like sodium and potassium, or neurotransmitters. These signals bind to specific receptors, setting off a chain reaction that leads to a cellular response.
In recent years, researchers have uncovered how disruptions in cell signaling contribute to diseases. For example, faulty signaling pathways are at the heart of conditions like diabetes, where insulin signaling fails, or Alzheimer’s, where neuronal communication breaks down. Understanding these pathways has led to targeted therapies, such as drugs that block overactive signals in cancer cells.
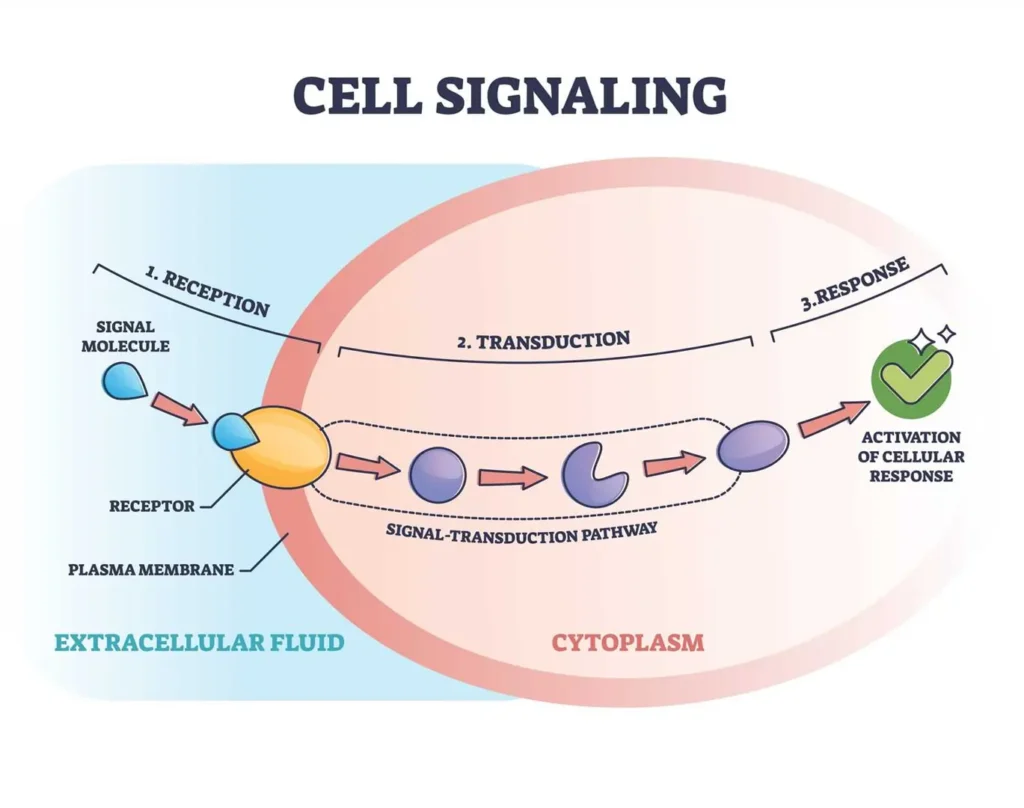
Stages of Cell Signaling
Cell signaling typically unfolds in a series of well-orchestrated stages, ensuring that the message is delivered accurately and efficiently. These stages form a pathway from signal reception to the final response and eventual shutdown.
First comes reception, where a signaling molecule, or ligand, binds to a specific receptor on the cell’s surface or inside the cell. Receptors act like locks, and ligands are the keys that fit perfectly.
Next is transduction, the relay phase. Once the ligand binds, it triggers a cascade of events, often involving phosphorylation (adding phosphate groups to proteins) or activation of enzymes. This step converts the external signal into an internal one that the cell can understand.
Amplification follows, where the signal gets boosted at multiple points. A single ligand might activate dozens of downstream molecules, ensuring even a weak signal elicits a strong response. This is crucial in scenarios like immune activation, where a tiny amount of pathogen can trigger a massive defense.
Then there’s integration, allowing cells to process multiple signals simultaneously. Cells don’t respond to signals in isolation; they weigh inputs from various sources to make informed decisions, much like how your brain integrates sights, sounds, and smells.
The cellular response is the payoff: changes in gene expression, metabolism, growth, or movement. For example, in muscle cells, signaling might lead to contraction during exercise.
Finally, termination wraps it up. Signals are deactivated by removing ligands, degrading messengers, or using phosphatases to reverse phosphorylation. This prevents constant activation, which could lead to exhaustion or disease.
Taxonomic Range of Cell Signaling
Cell signaling isn’t limited to complex life forms; it’s a universal feature across the tree of life, enabling coordination and adaptation in diverse organisms.
In bacteria, quorum sensing stands out as a prime example. Bacteria release signaling molecules called autoinducers, which accumulate as population density increases. Once a threshold is reached, they trigger behaviors like biofilm formation, which protects communities from antibiotics or harsh conditions. This has implications for treating infections, as disrupting quorum sensing could weaken bacterial defenses.
Archaea, ancient microbes thriving in extreme environments, use similar signaling to communicate and form communities. For instance, in hot springs, archaea signal to coordinate nutrient sharing or stress responses.
Protists, single-celled eukaryotes like amoebas or paramecia, rely on signaling for locomotion, feeding, and reproduction. In slime molds, starving cells signal each other to aggregate into a multicellular structure, showcasing early multicellular-like behavior.
In fungi, signaling regulates mating, where pheromones guide hyphal fusion, or stress responses, like in yeast adapting to nutrient scarcity by forming spores. Filamentation in pathogenic fungi, such as Candida, allows invasion of host tissues.
Plants use signaling extensively for growth and defense. Hormones like auxin direct root and shoot development, while jasmonic acid signals in response to insect attacks, prompting the release of protective compounds.
In animals, signaling reaches new heights of complexity. It’s vital for embryonic development, where gradients of signals determine body patterns, or in the nervous system, where electrical and chemical signals enable thought and movement.
Humans, as advanced animals, depend on signaling for homeostasis maintaining stable internal conditions like blood sugar levels immune responses, and organ function. Recent studies highlight how gut bacteria influence human signaling via the microbiome, affecting mood and immunity.
To illustrate the breadth, here’s a detailed table comparing cell signaling across taxa:
| Organism Group | Key Signaling Mechanism | Examples | Role in Survival |
|---|---|---|---|
| Bacteria | Quorum sensing via autoinducers | Biofilm formation in Pseudomonas aeruginosa | Coordinates group behaviors for protection and virulence |
| Archaea | Two-component systems | Methanogen communication in extreme heat | Adapts to harsh environments like deep-sea vents |
| Protists | Cyclic AMP pathways | Aggregation in Dictyostelium discoideum | Enables multicellular slug formation during starvation |
| Fungi | Pheromone signaling | Mating in Saccharomyces cerevisiae (yeast) | Facilitates reproduction and genetic diversity |
| Plants | Hormone signaling (e.g., ethylene) | Fruit ripening in tomatoes | Responds to environmental cues like light and drought |
| Animals | Neurotransmitter release | Synaptic transmission in neurons | Supports complex behaviors and sensory processing |
| Humans | Cytokine networks | Immune response to viruses | Maintains health by fighting infections and healing wounds |
This table underscores how signaling evolves from simple chemical exchanges in microbes to intricate networks in multicellular life.
Types of Cell Signaling
Cell signaling varies based on the signal’s nature, travel distance, and mechanism. Here’s a breakdown of the main types, each with unique roles.
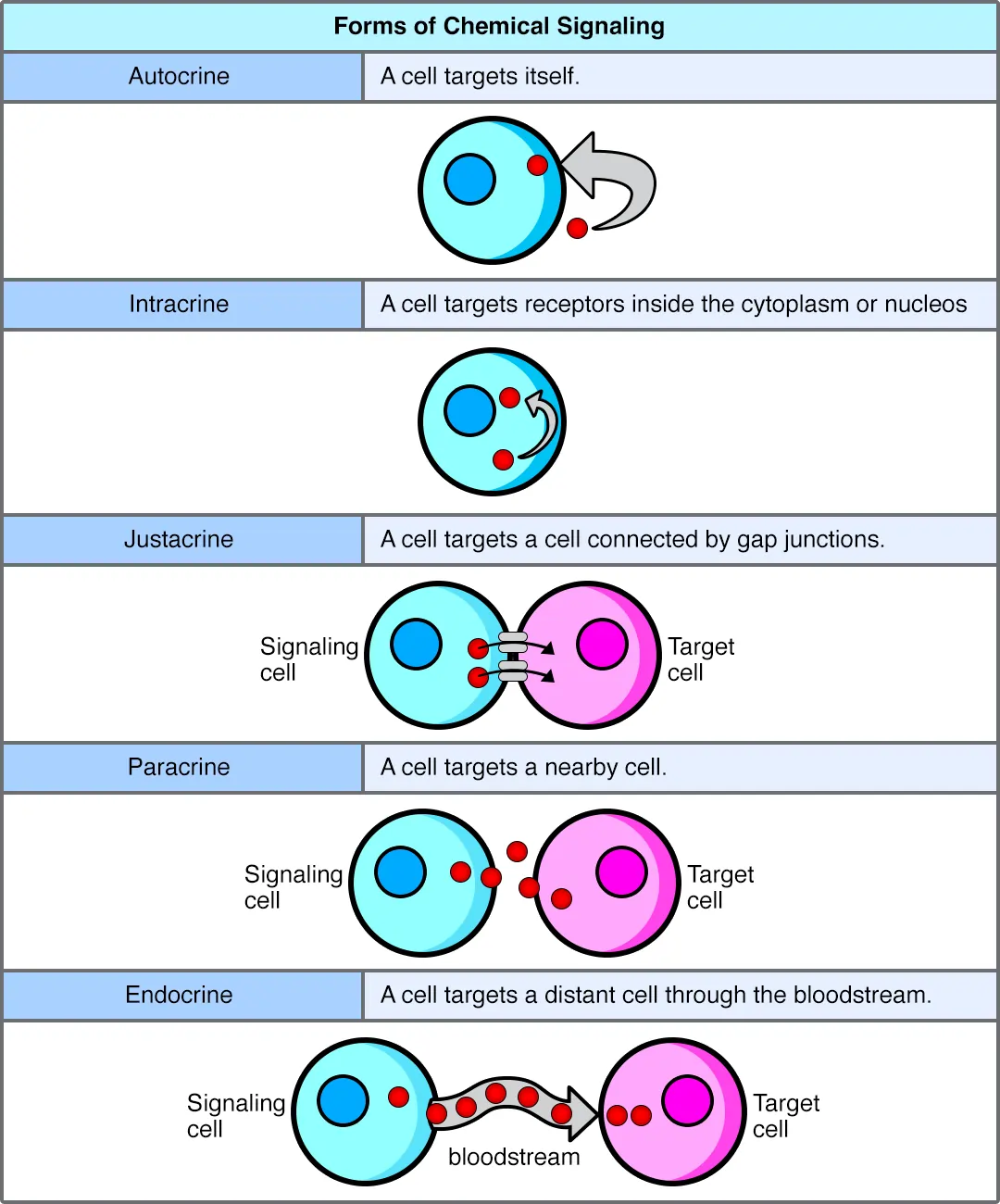
Autocrine signaling occurs when a cell signals itself. The cell releases molecules that bind to its own receptors, often promoting growth. In cancer, tumor cells use autocrine loops with growth factors like EGF to proliferate uncontrollably.
Paracrine signaling targets nearby cells. Signals diffuse through extracellular fluid to affect neighbors. Neurotransmitters in synapses or cytokines in inflammation exemplify this, coordinating local responses like wound healing.
Endocrine signaling involves long-distance travel via the bloodstream. Hormones from glands, such as insulin from the pancreas regulating glucose, reach distant targets. This maintains body-wide balance.
Juxtacrine signaling requires direct cell contact. Ligands on one cell’s membrane bind receptors on an adjacent cell. Notch signaling in development patterns tissues by inhibiting neighboring cells from the same fate.
Intracrine signaling happens entirely inside a cell. Ligands like steroid hormones cross the membrane and act on intracellular receptors, directly influencing gene expression without external input.
Contact-dependent signaling overlaps with juxtacrine, emphasizing physical touch. In immune synapses, T cells activate upon contacting antigen-presenting cells, crucial for adaptive immunity.
Synaptic signaling is specialized for neurons, where neurotransmitters cross tiny gaps called synapses. This enables rapid communication, like in reflexes.
Redox signaling involves electron transfers, often via reactive oxygen species (ROS). Once seen as harmful, ROS now recognized as messengers in processes like cell proliferation or apoptosis.
Additional types include mechanotransduction, where physical forces like stretch signal cells (e.g., in blood vessels sensing flow), and electrical signaling in excitable cells like heart muscle.
For a clearer view, consider this extensive table of types with examples:
| Type of Signaling | Description | Distance | Key Molecules | Real-World Example | Potential Disorders |
|---|---|---|---|---|---|
| Autocrine | Cell signals itself | Self | Cytokines, growth factors | Cancer cell proliferation | Autoimmune diseases like rheumatoid arthritis |
| Paracrine | Affects nearby cells | Short | Neurotransmitters, retinoic acid | Local immune response at injury site | Chronic inflammation in asthma |
| Endocrine | Travels via blood to distant cells | Long | Hormones like thyroid hormone | Regulation of metabolism | Endocrine disorders like hypothyroidism |
| Juxtacrine | Direct cell-cell contact | Contact | Membrane-bound ligands (e.g., Delta) | Embryonic tissue patterning | Developmental defects in congenital disorders |
| Intracrine | Within the same cell | Internal | Steroid hormones | Gene regulation by estrogen | Hormone-resistant cancers |
| Contact-Dependent | Physical interaction required | Contact | Integrins, cadherins | T cell activation by APCs | Immune deficiencies |
| Synaptic | Neuron-to-neuron via synapses | Short | Acetylcholine, dopamine | Nerve impulse transmission | Neurodegenerative diseases like Parkinson’s |
| Redox | Electron-based via oxidants | Varies | ROS, nitric oxide | Response to oxidative stress | Aging-related conditions |
This variety ensures cells can respond to diverse stimuli effectively.
Receptors in Cell Signaling
Receptors are the gatekeepers of signaling, binding ligands and initiating responses. They come in various forms, located on the surface, in the cytoplasm, or nucleus.
Cell surface receptors include G-protein coupled receptors (GPCRs), the largest family, involved in senses like vision (rhodopsin) and smell. Binding activates G-proteins, leading to second messengers like cAMP.
Receptor tyrosine kinases (RTKs) phosphorylate tyrosines upon ligand binding, activating pathways like MAPK for growth. Insulin receptors are RTKs; mutations cause diabetes.
Ion channel receptors open pores for ions, enabling fast responses. Nicotinic acetylcholine receptors at neuromuscular junctions trigger muscle contraction.
Intracellular receptors like nuclear receptors bind lipophilic ligands (e.g., glucocorticoids) and regulate transcription. Cytoplasmic receptors include those in MAPK pathways, relaying signals without entering the nucleus.
Enzyme-linked receptors such as receptor guanylyl cyclases produce cGMP, a messenger in vision and vasodilation. Histidine kinase receptors in bacteria phosphorylate histidines for two-component systems.
Pattern recognition receptors like Toll-like receptors (TLRs) detect pathogens, sparking immunity. Mutations in TLRs link to increased infection risk.
Other categories include adhesion receptors (integrins for cell-matrix interactions) and cytokine receptors (for immune modulation).
Recent advances show receptors can form complexes, enhancing specificity. For instance, in CRISPR-edited models, altering receptors has potential for treating signaling-related diseases.
Here’s a comprehensive table of receptor types:
| Receptor Category | Subtype | Location | Function | Ligands | Associated Pathways | Diseases Linked |
|---|---|---|---|---|---|---|
| Cell Surface | GPCR | Membrane | Activate G-proteins | Hormones, neurotransmitters | cAMP, IP3/DAG | Hypertension, schizophrenia |
| Cell Surface | RTK | Membrane | Tyrosine phosphorylation | Growth factors (EGF) | MAPK, PI3K | Cancer, diabetes |
| Cell Surface | Ion Channel | Membrane | Ion flux | Acetylcholine | Electrical signaling | Myasthenia gravis |
| Intracellular | Nuclear | Nucleus/Cytoplasm | Transcription regulation | Steroids, thyroid hormones | Gene expression | Hormone imbalances |
| Intracellular | Cytoplasmic | Cytoplasm | Signal relay | Various kinases | JAK-STAT, MAPK | Inflammatory diseases |
| Enzyme-Linked | Guanylyl Cyclase | Membrane | cGMP production | Atrial natriuretic peptide | Vasodilation | Heart failure |
| Enzyme-Linked | Histidine Kinase | Membrane (mostly prokaryotes) | Phosphorelay | Environmental signals | Two-component systems | Bacterial virulence |
| Pattern Recognition | TLR | Membrane/Endosomes | Pathogen detection | LPS, dsRNA | NF-κB | Sepsis, autoimmunity |
| Other | Adhesion (Integrins) | Membrane | Cell attachment | Extracellular matrix | Focal adhesion kinase | Thrombosis |
| Other | Cytokine | Membrane | Immune modulation | Interleukins | JAK-STAT | Rheumatoid arthritis |
This table highlights the diversity and importance of receptors.
Functions of Cell Signaling
Cell signaling drives countless functions, from basic survival to complex behaviors.
Communication between cells allows tissues to work as units. In the heart, signaling synchronizes contractions for pumping blood.
Development and differentiation guide embryonic cells to become specialized, like stem cells turning into neurons via Wnt signaling.
Immune defense relies on signals to detect and eliminate threats. Cytokines rally immune cells during infections.
Cell growth and division are regulated to prevent uncontrolled proliferation. The cell cycle checkpoints use signaling to pause for repairs.
Metabolism adjustments, like glucagon signaling to release glucose during fasting, maintain energy balance.
Additional functions include apoptosis (programmed death) to remove damaged cells, migration in wound healing, and sensory perception, where light signals trigger vision.
In diseases, aberrant signaling causes issues: overactive in cancer, underactive in neurodegeneration. Therapies like monoclonal antibodies target these pathways.
To expand, signaling evolves: simple in prokaryotes for survival, complex in eukaryotes for multicellularity. Recent research links signaling to epigenetics, where signals alter gene accessibility without changing DNA.
In summary, cell signaling is life’s conductor, orchestrating harmony across scales. As we uncover more, it promises breakthroughs in medicine and biotechnology.
Related Articles
- Contact-Dependent Signaling: Direct Cell Communication in Animals and Plants
- Synaptic Signaling: Mechanisms, Learning, and Neurological Disorders
- Autocrine Signaling: Mechanisms, Roles, and Therapeutic Potential
- Intracrine Signaling: Mechanisms, Health Roles, and Therapeutic Potential
- Paracrine Signaling: Definition, Mechanism, and Key Examples
- Endocrine Signaling: Mechanisms, Types, and Role in Human Health
- Juxtacrine Signaling: Mechanisms, Functions, and Biological Roles
- Redox Signaling: Mechanisms, Health Impact, and Disease Connections
Frequently Asked Questions
FAQ 1: What is cell signaling and why is it important?
Cell signaling is the process by which cells communicate with each other or within themselves to carry out essential tasks like growth, division, or responding to environmental changes. Think of it as the body’s internal messaging system, where signals like hormones or neurotransmitters act as messages, and receptors on or inside cells act as receivers. This communication ensures that cells work together to maintain health, respond to threats, and perform functions like healing wounds or fighting infections.
Without cell signaling, life as we know it wouldn’t exist. For example, in humans, insulin signals cells to absorb glucose for energy, maintaining blood sugar levels. In plants, signaling helps them respond to sunlight or pests. Even single-celled organisms like bacteria use signaling to coordinate group behaviors, such as forming protective biofilms. Disruptions in signaling can lead to serious conditions like cancer, diabetes, or autoimmune diseases, making it a critical area of study for developing new treatments.
FAQ 2: What are the main stages of cell signaling?
Cell signaling follows a series of steps to ensure messages are delivered and acted upon correctly. The process begins with reception, where a signaling molecule, or ligand, binds to a specific receptor on a cell’s surface or inside it. This binding is highly specific, like a key fitting a lock, ensuring the right signal triggers the right response.
Next comes transduction, where the signal is converted into a form the cell can process. This often involves a cascade of chemical reactions, such as phosphorylation, where enzymes add phosphate groups to proteins to activate them. The signal may then undergo amplification, boosting its strength to elicit a robust response. Integration allows cells to process multiple signals at once, leading to a tailored cellular response, like gene expression or movement. Finally, termination shuts down the signal to prevent overstimulation, using mechanisms like ligand removal. These stages ensure precise and efficient communication, vital for processes like immune responses or tissue development.
FAQ 3: What are the different types of cell signaling?
Cell signaling comes in several forms, each suited to specific distances and purposes. Autocrine signaling occurs when a cell signals itself, often seen in cancer cells that release growth factors to fuel their own division. Paracrine signaling affects nearby cells, like neurotransmitters triggering responses in adjacent neurons during a reflex. Endocrine signaling involves hormones traveling through the bloodstream to distant cells, such as thyroid hormones regulating metabolism.
Other types include juxtacrine signaling, requiring direct cell contact, as in embryonic development, and intracrine signaling, where signals act within a single cell, like steroid hormones altering gene expression. Synaptic signaling is specific to neurons, enabling rapid communication in the nervous system. Redox signaling, involving molecules like reactive oxygen species, regulates processes like cell growth. Each type plays a unique role, ensuring cells respond appropriately to diverse stimuli, from local infections to body-wide hormonal changes.
FAQ 4: How does cell signaling work in different organisms?
Cell signaling is universal across life forms, from simple bacteria to complex humans. In bacteria, quorum sensing allows them to communicate using chemical signals called autoinducers, coordinating behaviors like biofilm formation to resist antibiotics. Archaea, living in extreme environments, use similar signaling to adapt to heat or acidity, ensuring survival.
In protists, single-celled eukaryotes, signaling drives movement and reproduction, like slime molds aggregating during starvation. Fungi use signaling for mating and stress responses, such as yeast forming spores under nutrient scarcity. Plants rely on hormones like auxin for growth or jasmonic acid to fend off pests. In animals and humans, signaling is highly complex, supporting immune responses, brain function, and embryonic development. For example, human cytokine signaling orchestrates immune defenses against viruses. This diversity highlights how signaling adapts to each organism’s needs, enabling survival and coordination across the tree of life.
FAQ 5: What are the roles of receptors in cell signaling?
Receptors are specialized proteins that detect signaling molecules and initiate cellular responses. Located on cell surfaces or inside cells, they ensure signals are received accurately. G-protein coupled receptors (GPCRs), for instance, bind signals like hormones and activate internal proteins to trigger responses, playing a role in vision and smell. Receptor tyrosine kinases (RTKs) phosphorylate proteins to drive growth, as seen with insulin receptors.
Ion channel receptors allow ions to flow, enabling fast responses like muscle contractions. Inside cells, nuclear receptors regulate gene expression when bound by steroid hormones, while cytoplasmic receptors relay signals without entering the nucleus. Toll-like receptors (TLRs) detect pathogens, sparking immune responses. Each receptor type is finely tuned, ensuring cells respond appropriately. Faulty receptors can cause diseases like diabetes or infections, making them key targets for therapies.
FAQ 6: How does cell signaling contribute to human health?
Cell signaling is crucial for maintaining homeostasis, the body’s ability to keep conditions like temperature and blood sugar stable. For example, insulin signaling regulates glucose uptake, preventing diabetes. In the immune system, cytokine signaling coordinates responses to infections, ensuring pathogens are eliminated efficiently.
Signaling also drives cell growth and repair, essential for healing wounds or regenerating tissues. In the nervous system, synaptic signaling enables thought, memory, and movement. However, when signaling goes wrong, it can lead to diseases. Overactive signaling fuels cancer, while underactive pathways contribute to neurodegenerative disorders like Alzheimer’s. Advances in understanding signaling have led to treatments, such as drugs targeting faulty pathways in leukemia or monoclonal antibodies for autoimmune diseases, highlighting its role in health and medicine.
FAQ 7: What happens when cell signaling fails?
When cell signaling malfunctions, it can disrupt critical processes, leading to disease. For instance, in cancer, autocrine signaling loops cause cells to grow uncontrollably by producing their own growth signals. In diabetes, defective insulin signaling prevents cells from absorbing glucose, causing high blood sugar levels.
Faulty immune signaling can result in autoimmune diseases, where the body attacks itself, as seen in rheumatoid arthritis. In the brain, disrupted synaptic signaling contributes to conditions like Parkinson’s or depression. Even infections can worsen if Toll-like receptors fail to detect pathogens. These failures underscore why signaling pathways are tightly regulated and why researchers focus on them for developing targeted therapies, such as drugs to correct signaling errors in chronic diseases.
FAQ 8: How is cell signaling involved in development and differentiation?
During development, cell signaling guides how cells specialize into specific types, a process called differentiation. For example, in embryos, Notch signaling determines whether a cell becomes a neuron or a skin cell by interacting with neighboring cells. This ensures tissues and organs form correctly.
Signaling also controls cell growth and positioning. In humans, gradients of signaling molecules like Sonic Hedgehog shape limbs and organs during fetal development. In plants, auxin signaling directs root and shoot growth, ensuring proper structure. Errors in developmental signaling can lead to congenital disorders, such as heart defects. Recent studies show that manipulating signaling pathways in stem cells could advance regenerative medicine, allowing scientists to grow specific tissues for transplants.
FAQ 9: How does cell signaling differ between prokaryotes and eukaryotes?
Prokaryotes, like bacteria, use simple signaling systems suited to their single-celled nature. Quorum sensing is a key example, where bacteria release chemicals to coordinate group activities like biofilm formation. These systems are often two-component, involving a sensor and a response regulator, allowing quick adaptation to environmental changes.
Eukaryotes, including plants and animals, have more complex signaling due to their multicellular structure. They use diverse pathways, like GPCR or RTK signaling, to manage intricate processes such as immune responses or organ development. For instance, human neurons rely on synaptic signaling for rapid communication, while plants use hormone signaling for growth. Eukaryotic signaling often involves amplification and integration of multiple signals, enabling nuanced responses. This complexity reflects the need for coordination in multicellular organisms, unlike the simpler survival-driven signaling in prokaryotes.
FAQ 10: How is cell signaling being used in medical research?
Cell signaling is a hot topic in medical research because it underpins many diseases and offers pathways for treatment. Researchers study signaling pathways to understand how they go wrong in conditions like cancer, where overactive RTK signaling drives tumor growth. Drugs like tyrosine kinase inhibitors block these pathways, slowing cancer progression.
In immunotherapy, scientists manipulate cytokine signaling to boost immune responses against tumors. Signaling research also aids regenerative medicine; for example, tweaking Wnt signaling in stem cells could help grow organs for transplants. Additionally, understanding redox signaling has led to therapies targeting oxidative stress in aging-related diseases. By unraveling signaling networks, researchers are developing precision medicines that target specific pathways, minimizing side effects and improving outcomes for patients.
FAQ 11: How has cell signaling evolved across different organisms?
Cell signaling has undergone remarkable evolution, adapting from simple mechanisms in ancient unicellular organisms to complex networks in multicellular life forms. In early prokaryotes, signaling was basic, often involving direct responses to environmental cues like nutrients or stresses through two-component systems. These systems, still present in bacteria today, consist of a sensor kinase that detects changes and a response regulator that alters gene expression or behavior. As life evolved, these foundational elements were conserved but expanded, allowing for more sophisticated communication essential for multicellularity.
The transition to eukaryotes brought significant advancements. Around 1.6 to 0.6 billion years ago, plants and animals diverged, each developing multicellularity independently. In animals, pathways like Wnt, Notch, and Hedgehog emerged to coordinate development, tissue patterning, and homeostasis. These pathways rely on intricate protein interactions and second messengers, enabling precise control over cell fate. For instance, the Wnt pathway, absent in plants but crucial in animals, stabilizes β-catenin to regulate transcription, influencing everything from embryonic development to cancer. Plants, evolving separately, developed unique systems like hormone signaling with auxin and ethylene, which control growth and responses to light or gravity.
Crosstalk between pathways also evolved, enhancing adaptability. In modern organisms, signaling networks integrate multiple inputs, with shared components like G-protein-coupled receptors (GPCRs) appearing in both lineages but diverging in function. GPCRs, the largest receptor family, handle senses in animals but environmental sensing in plants. Evolutionary pressures, such as the need for rapid responses in mobile animals versus sessile plants, shaped these differences. Mutations in proto-oncogenes or tumor suppressors often disrupt evolved balances, leading to diseases like cancer, where hyperactive signaling mimics ancient unregulated growth.
Future research may uncover more about how environmental factors drove signaling evolution, potentially revealing therapeutic targets. Understanding this progression highlights signaling’s role in life’s complexity, from bacterial quorum sensing to human neural networks.
FAQ 12: What are key cell signaling pathways involved in cancer?
Cell signaling pathways play a pivotal role in cancer development, where dysregulation leads to uncontrolled growth, invasion, and metastasis. Understanding these pathways is crucial for targeted therapies.
| Pathway | Description | Key Components | Role in Cancer | Therapeutic Targets | Examples of Cancers |
|---|---|---|---|---|---|
| PI3K/AKT/mTOR | Regulates cell growth, survival, and metabolism. Activated by growth factors binding to RTKs. | PI3K, AKT, mTOR, PTEN (inhibitor) | Promotes proliferation, inhibits apoptosis; mutations in PTEN lead to overactivation. | mTOR inhibitors (e.g., rapamycin), PI3K inhibitors. | Breast, prostate, colorectal cancers. |
| Ras/MAPK (ERK) | Controls cell proliferation and differentiation. Activated by RTKs like EGFR. | Ras, Raf, MEK, ERK | Mutations in Ras cause constitutive activation, driving tumor growth. | MEK inhibitors (e.g., trametinib), BRAF inhibitors. | Lung, pancreatic, melanoma. |
| Wnt/β-catenin | Involved in cell fate and proliferation. | Wnt ligands, Frizzled receptors, β-catenin, APC. | Aberrant activation leads to nuclear β-catenin accumulation, promoting oncogenes. | Wnt inhibitors, β-catenin stabilizers. | Colorectal, liver cancers. |
| Notch | Regulates cell differentiation and angiogenesis. | Notch receptors, Delta/Jagged ligands. | Overactivation enhances stemness and resistance; mutations common in leukemias. | Gamma-secretase inhibitors. | T-cell leukemia, breast cancer. |
| Hedgehog | Controls tissue patterning and stem cell maintenance. | Hedgehog ligands, Patched, Smoothened. | Drives cancer stem cell renewal; mutations in Patched activate pathway. | Smoothened inhibitors (e.g., vismodegib). | Basal cell carcinoma, medulloblastoma. |
| TGF-β/Smad | Dual role in tumor suppression and promotion. | TGF-β ligands, Smad proteins. | Early suppression of growth; later promotes EMT and metastasis. | TGF-β receptor inhibitors. | Pancreatic, colorectal cancers. |
| NF-κB | Regulates inflammation and immune response. | NF-κB subunits, IκB inhibitors. | Chronic activation leads to inflammation-driven carcinogenesis. | IKK inhibitors. | Lymphoma, multiple myeloma. |
| JAK/STAT | Mediates cytokine signaling for growth and immunity. | JAK kinases, STAT transcription factors. | Persistent activation promotes survival and proliferation. | JAK inhibitors (e.g., ruxolitinib). | Hematologic malignancies, solid tumors. |
These pathways often crosstalk, amplifying oncogenic effects. For instance, PI3K/AKT can inhibit apoptosis while activating NF-κB inflammation. Targeting multiple pathways simultaneously is a growing strategy to overcome resistance.
FAQ 13: What are second messengers in cell signaling, and how do they function?
Second messengers are crucial intracellular molecules that amplify and propagate signals from cell surface receptors to effector proteins, enabling rapid cellular responses. These small, non-protein compounds include cyclic nucleotides like cAMP and cGMP, lipids such as IP3 and DAG, ions like Ca2+, and gases including nitric oxide.
- cAMP (cyclic adenosine monophosphate): Produced by adenylyl cyclase from ATP upon GPCR activation. It activates protein kinase A (PKA), which phosphorylates targets to regulate metabolism, gene expression, and ion channels. In heart cells, cAMP boosts contraction; dysregulation links to heart failure.
- IP3 (inositol trisphosphate) and DAG (diacylglycerol): Generated by phospholipase C from PIP2. IP3 releases Ca2+ from ER stores, while DAG activates PKC for processes like inflammation and growth. In immune cells, this pathway drives cytokine release.
- Ca2+ (calcium ions): Acts as a versatile messenger, entering via channels or released from stores. Binds calmodulin to activate enzymes, influencing muscle contraction, neurotransmitter release, and apoptosis. Elevated Ca2+ in neurons triggers synaptic plasticity.
- cGMP (cyclic guanosine monophosphate): Synthesized by guanylyl cyclase, often in response to nitric oxide. Activates PKG for vasodilation and smooth muscle relaxation; PDE5 inhibitors like sildenafil enhance this for treating erectile dysfunction.
- Other messengers: Nitric oxide diffuses freely, activating guanylyl cyclase for vascular tone. Reactive oxygen species (ROS) signal in redox pathways, but excess causes oxidative stress linked to aging.
These messengers ensure signal specificity through compartmentalization and transient activation, with parameters like concentration gradients dictating outcomes. Dysfunctions contribute to diseases like cancer and neurodegeneration.
FAQ 14: What is crosstalk between cell signaling pathways, and why is it important?
Crosstalk refers to interactions between different signaling pathways, where activation of one influences another, leading to integrated cellular responses. This isn’t random noise but a deliberate mechanism for fine-tuning biology, allowing cells to process multiple inputs simultaneously.
In direct crosstalk, pathways share components; for example, in yeast MAPK pathways, scaffolding proteins like Ste5 prevent unwanted interactions, but shared kinases enable coordination. Indirect crosstalk occurs via feedback loops or gene regulation, as seen in brassinosteroid and immune signaling in plants sharing BAK1 receptor, where dosage affects immunity.
Importance lies in adaptability: crosstalk enables synergism (amplified responses), antagonism (balancing signals), or co-regulation (additive effects). In cancer, dysregulated crosstalk like PI3K/AKT activating NF-κB promotes inflammation and survival. In immunity, TLR-IκB-NFκB integrates with interferon pathways via IRAK upregulation.
Evolutionarily, crosstalk enhances diversity; simulations show it allows varied outputs from limited signals. Pathologically, imbalances cause diseases; understanding crosstalk could improve therapies by targeting hubs like BAK1.
Future studies may map dynamic crosstalk networks using single-cell proteomics, revealing context-specific roles.
FAQ 15: How does cell signaling regulate stem cell differentiation?
Cell signaling is central to stem cell differentiation, guiding pluripotent cells toward specific lineages through orchestrated pathways.
| Pathway | Key Signals | Role in Differentiation | Cell Types Influenced | Examples of Regulators | Diseases Linked |
|---|---|---|---|---|---|
| Wnt/β-catenin | Wnt ligands | Maintains pluripotency or promotes mesendoderm; inhibits neural fates. | ESCs, MSCs, HSCs | GSK-3 inhibitors like CHIR99021. | Cancer, osteoporosis. |
| BMP/Smad | BMP proteins | Drives mesoderm, bone formation; inhibits neural differentiation. | MSCs, ESCs | Noggin (antagonist). | Skeletal disorders. |
| Notch | Delta/Jagged ligands | Regulates binary fate choices; promotes glial over neuronal in CNS. | NSCs, HSCs | Gamma-secretase inhibitors. | Leukemia, Alzheimer’s. |
| FGF/MAPK | FGFs | Supports pluripotency; directs ectoderm/neural lineages. | ESCs, iPSCs | PD0325901 (MEK inhibitor). | Developmental defects. |
| TGF-β/Activin | TGF-β, Nodal | Promotes endoderm; maintains pluripotency via Smad2/3. | ESCs, iPSCs | SB431542 (inhibitor). | Fibrosis, cancer. |
| Hedgehog | Shh | Patterns tissues; promotes ventral fates in neural tube. | NSCs, MSCs | Cyclopamine (inhibitor). | Birth defects, medulloblastoma. |
| JAK/STAT | LIF (in mice) | Inhibits differentiation; maintains mESC pluripotency. | ESCs | Ruxolitinib (JAK inhibitor). | Immune disorders. |
Crosstalk, like Wnt enhancing BMP effects, ensures precise control. Small molecules modulate these for regenerative medicine.
FAQ 16: What techniques are used to study cell signaling?
Studying cell signaling requires diverse methods to capture dynamic interactions, from molecular to systems levels.
- Western blotting and immunoprecipitation: Detect protein activation via phosphorylation; pull-down assays reveal interactions. Useful for pathway mapping, but limited to snapshots.
- Fluorescence microscopy (FRET, FRAP): FRET measures proximity (<10 nm) for protein interactions in live cells; FRAP assesses mobility. Enables real-time visualization, e.g., ERK activation.
- Mass spectrometry: Quantifies phosphoproteomes; identifies PTMs and networks. High-throughput, but requires expertise.
- Flow cytometry: Analyzes signaling in populations; detects phosphorylated proteins via antibodies. Good for heterogeneity studies.
- Single-cell RNA-seq: Reveals transcriptional responses; infers pathways from gene expression. Captures cell variability.
- CRISPR-based screens: Knocks out genes to identify regulators; forward genetics for pathway discovery.
- Biosensors and optogenetics: Engineered probes report second messengers like Ca2+; light-activated tools control signaling spatially.
- Computational modeling: Simulates networks; predicts crosstalk outcomes. Integrates multi-omics data.
These techniques, often combined, advance understanding from basic mechanisms to therapeutic targets.
FAQ 17: How does cell signaling change with aging, and what are the implications?
Aging disrupts cell signaling, leading to impaired homeostasis and increased disease risk. Pathways like IIS, mTOR, and AMPK shift, promoting senescence and inflammation.
In young cells, balanced signaling maintains repair; with age, chronic activation of pro-inflammatory paths like NF-κB drives inflammaging, exacerbating conditions like arthritis. Insulin/IGF-1 signaling declines, reducing FOXO activity and antioxidant defenses, heightening oxidative stress.
Mitochondrial signaling falters; mtDNA mutations accumulate, releasing DAMPs that activate cGAS-STING, fueling SASP and systemic inflammation. This contributes to neurodegeneration, as in Alzheimer’s, where amyloid disrupts synaptic signaling.
Epigenetic changes alter receptor expression; histone modifications dysregulate Wnt, promoting fibrosis. Interventions like CR activate SIRT1, restoring balance and extending lifespan in models.
Aging also affects intercellular communication; senescent cells secrete SASP factors, propagating dysfunction. Future therapies may target hubs like mTOR with rapamycin to mitigate these effects.
FAQ 18: What environmental factors influence cell signaling?
Environmental factors profoundly impact cell signaling, modulating pathways to adapt or cause pathology.
- Nutrients and diet: Glucose activates insulin signaling; caloric restriction boosts AMPK/SIRT1 for longevity. High-fat diets induce inflammation via NF-κB.
- Temperature: Heat shock activates HSPs via HSF1; cold stress engages adrenergic signaling for thermogenesis.
- Light: In plants, phytochromes sense red light, activating growth paths; in animals, circadian rhythms via melanopsin affect melatonin signaling.
- Toxins and pollutants: Heavy metals disrupt Ca2+ signaling; pesticides alter hormone receptors, leading to endocrine disruption.
- Stressors like hypoxia: Low oxygen activates HIF-1α, reprogramming metabolism and angiogenesis.
- Microbiota: Gut bacteria produce metabolites influencing immune signaling via TLRs.
- Physical forces: Shear stress in vessels activates mechanosensors like integrins, regulating vascular tone.
These factors highlight signaling’s role in environmental adaptation, with imbalances linking to diseases like cancer.
FAQ 19: What are the main differences in cell signaling between plants and animals?
Plant and animal signaling share eukaryotic roots but diverged, reflecting lifestyles: sessile plants vs. mobile animals.
| Aspect | Plants | Animals | Key Differences | Shared Elements |
|---|---|---|---|---|
| Major Pathways | Auxin, ethylene, cytokinin; rely on receptor kinases. | Wnt, Notch, Hedgehog; use GPCRs extensively. | Plants lack Wnt/Notch; animals lack auxin systems. | Both use MAPK, Ca2+ signaling. |
| Receptors | Mostly serine/threonine kinases; fewer GPCRs. | Abundant GPCRs, RTKs. | Plants emphasize enzyme-linked; animals G-protein linked. | Nuclear receptors for steroids/hormones. |
| Second Messengers | IP3, cGMP, ROS; no cAMP definitively. | cAMP, IP3, Ca2+, NO. | Plants use gases like ethylene; animals neurotransmitters. | Ca2+ universal. |
| Intercellular | Plasmodesmata for direct transfer; hormones diffuse/air. | Gap junctions, synapses; endocrine via blood. | Plants use symplastic paths; animals electrical/chemical synapses. | Paracrine/endocrine common. |
| Environmental Response | Light (phytochromes), gravity, pathogens. | Sensory (vision, touch), hormones. | Plants evolved for immobility; animals for movement. | Stress responses conserved. |
| Evolution | Independent multicellularity; no nerves/muscles. | Shared ancestor; complex neural nets. | Diverged 1.6-0.6 BYA; separate adaptations. | Conserved basics like two-component systems. |
These distinctions underscore evolutionary adaptations to environments.
FAQ 20: What are future directions in cell signaling research?
Cell signaling research is poised for transformative advances, driven by technology and interdisciplinary approaches. Single-cell analyses will dominate, revealing heterogeneity in signaling networks missed by bulk methods. Techniques like spatial transcriptomics and multiplex imaging will map interactions in tissues, uncovering microenvironment influences.
Therapeutic targeting will evolve; understanding crosstalk could enable multi-pathway inhibitors, reducing resistance in cancer. AI and machine learning will model complex dynamics, predicting outcomes from perturbations.
Stem cell and regenerative medicine will benefit from decoding differentiation signals, potentially via optogenetics for precise control. Aging research will focus on restoring youthful signaling, targeting SASP or mitochondrial pathways.
Environmental impacts, like pollutants disrupting endocrine signaling, will gain attention amid climate change. Finally, synthetic biology may engineer novel pathways for bioengineering applications, from biosensors to therapeutic cells.
Read These Articles in Detail
- The Cell: The Fundamental Building Block of Life
- Prokaryotic Cells vs. Eukaryotic Cells: A Detailed Exploration
- Prokaryotic Cells: Definition, Anatomy, Functions, and Their Importance
- Eukaryotic Cells Explained: Definition, Structure, Functions, and Importance
- The Cell Membrane: Structure, Functions, and Evolutionary Significance
- The Cell Wall: Structure, Composition, Functions, and Evolutionary Significance
- Endomembrane System: A Detailed Exploration of the Cellular Machinery of Life
- Mitochondria: A Comprehensive Guide to Their Structure and Functions
- The Cell Envelope: The Protective Shield of Prokaryotic Cells
- Ribosomes: Definition, Structure, and Functions
- Endoplasmic Reticulum: Structure, Types, and Functions Explained
- The Golgi Apparatus: Structure, Functions, and Cellular Importance
- Cytoplasm and Nucleus: Structure, Functions, Differences, and Examples
- What Is Cell Signaling? Structure, Types, and Functions Explained
- Three Main Parts of a Nucleotide: Structure and Functions
- Enzymes: Definition, Structure, Classification, and Functions
- DNA: Structure, Function, Discovery, and Modern Applications
- DNA Replication: Steps, Process, and Genetic Importance
- Properties of DNA: Structure, Physical, and Chemical Characteristics
- DNA Packaging: How DNA Fits into the Nucleus of a Cell
- R-Factors and Plasmids: How Bacteria Develop Antibiotic Resistance
- Chromosomes: Structure, Types, Functions, and Role in Genetics
- Genetic Material: DNA, RNA, and Their Role in Heredity and Evolution
- Deoxyribose and Ribose: Structure and Function in DNA and RNA
- Differences Between Genes and DNA: Definitions, Structures, and Functions
- RNA: Definition, Structure, Types, Functions, and Its Role in Life
Acknowledgement
The creation of the article “What Is Cell Signaling? Structure, Types, and Functions Explained” was made possible through the wealth of knowledge available from several reputable sources. These platforms provided invaluable insights into the intricate mechanisms of cell signaling, its evolutionary significance, and its applications in health and disease. Their comprehensive and accessible information helped shape a detailed and accurate exploration of this critical biological process.
Below are the key sources that contributed to the article:
- Nature (www.nature.com): Offered in-depth reviews and primary research on signaling pathways, particularly in cancer and development.
- ScienceDirect (www.sciencedirect.com): Provided detailed studies on receptor biology, second messengers, and plant signaling.
- PubMed (pubmed.ncbi.nlm.nih.gov): Supplied peer-reviewed articles on signaling in aging, immunity, and therapeutic advancements.
- Cell Press (www.cell.com): Contributed cutting-edge research on crosstalk, stem cell differentiation, and signaling techniques.
- Annual Reviews (www.annualreviews.org): Delivered comprehensive overviews on evolutionary and environmental aspects of signaling.

