DNA replication stands as one of the most remarkable processes in biology, ensuring that every cell in our body carries the exact blueprint of life. This intricate mechanism allows cells to duplicate their genetic material with astonishing accuracy, passing on traits from one generation to the next. Whether you’re a student diving into molecular biology or simply curious about how life perpetuates itself, understanding DNA replication reveals the elegance of nature’s design.
In this extensive article, we’ll explore the fundamentals, step-by-step processes, key players like enzymes, differences across organisms, and even the broader implications for health and evolution. We’ll break it down into digestible sections with examples, bullet points, and tables to make complex ideas feel approachable and natural.
Table of Contents
What is DNA Replication?
DNA replication is the biological process where a cell makes an exact copy of its DNA. DNA, or deoxyribonucleic acid, serves as the genetic material in most living organisms, storing instructions for building and maintaining life. This duplication happens right before a cell divides, ensuring each new cell gets a full set of genetic information. It’s like photocopying a massive instruction manual so both daughter cells can function properly.
The process is semi-conservative, meaning each new DNA molecule consists of one original strand and one newly synthesized strand. This was brilliantly demonstrated through experiments where scientists labeled DNA with heavy isotopes and observed how the strands separated and reformed. Imagine unzipping a zipper: the two halves split, and each serves as a template for a new matching half.
DNA replication occurs during the S phase of the cell cycle in eukaryotic cells, which are those with a nucleus, like in animals and plants. In prokaryotic cells, such as bacteria, it happens in the cytoplasm and can start almost anytime the cell needs to divide. The entire operation is orchestrated by a suite of enzymes and proteins working in harmony, preventing chaos in the genetic code.
Why does this matter? Without accurate replication, cells couldn’t grow, repair tissues, or reproduce. Errors here can lead to mutations, which might cause diseases or drive evolution. For instance, in rapidly dividing cells like those in our skin or gut, replication happens millions of times daily, showcasing the robustness of this system.
The Importance of DNA Replication
DNA replication isn’t just a cellular chore; it’s fundamental to life itself. It enables the inheritance of genetic traits, allowing parents to pass on characteristics like eye color or height to their offspring. During cell division, whether mitosis for growth or meiosis for reproduction, replication ensures genetic continuity.
Beyond inheritance, replication plays a crucial role in repairing damaged DNA. Environmental factors like UV radiation or chemicals can harm DNA strands, and replication mechanisms help fix these issues to maintain cellular health. Think of it as a built-in backup system that keeps the genetic library intact.
Moreover, this process is vital for development and growth. In a growing embryo, cells divide rapidly, each requiring a complete DNA copy to differentiate into specialized tissues like muscles or nerves. Genetic variations arising from minor replication errors contribute to biodiversity, helping species adapt to changing environments over generations.
On the flip side, when replication goes awry, it can lead to serious problems. Uncontrolled replication in cancer cells, for example, allows tumors to grow unchecked. Understanding replication has led to breakthroughs in medicine, such as targeted therapies that inhibit faulty replication in diseased cells.
In biotechnology, scientists harness replication principles to amplify DNA for research, forensics, or genetic engineering. Techniques like PCR (polymerase chain reaction) mimic natural replication to produce millions of DNA copies from a tiny sample, revolutionizing fields from crime-solving to personalized medicine.
Historical Background of DNA Replication
The story of DNA replication begins in the mid-20th century, building on the discovery of DNA’s structure. In 1953, James Watson and Francis Crick proposed the double-helix model, suggesting that the complementary base pairing—adenine with thymine, guanine with cytosine—could explain how DNA copies itself. This idea sparked intense research into the mechanics of replication.
A pivotal moment came in 1958 with the Meselson-Stahl experiment. Using bacteria grown in media with heavy nitrogen isotopes, they showed that replication is semi-conservative. After one generation, DNA was hybrid—half heavy, half light—and after two, it split into hybrid and fully light forms. This elegant proof confirmed that strands separate and each templates a new one.
Further discoveries identified key enzymes. In 1976, DNA helicase was isolated from E. coli, revealing how the helix unwinds. Advances in the 1980s and 1990s mapped out eukaryotic replication, highlighting complexities like multiple origins. Today, ongoing research explores replication’s links to aging and disease, using advanced imaging to watch it in real time.
These historical insights underscore how replication went from a hypothesis to a well-understood process, influencing everything from evolutionary biology to synthetic life creation.
Models of DNA Replication
Before the semi-conservative model was proven, scientists debated several theories on how DNA duplicates.
- Conservative Model: In this idea, the original DNA remains intact, and a completely new copy is made alongside it. It’s like keeping the master document and printing a fresh one. However, experiments showed this doesn’t happen, as daughter molecules always contain parts of the parent.
- Semi-Conservative Model: The accepted one, where the double helix unwinds, and each strand acts as a template for a new complementary strand. This results in two molecules, each with one old and one new strand. It’s efficient and explains genetic stability.
- Dispersive Model: Here, the original DNA breaks into pieces, and new segments fill in, mixing old and new throughout. This was disproven by density gradient experiments showing clear banding patterns inconsistent with fragmentation.
Understanding these models helps appreciate why semi-conservative replication is ideal: it minimizes errors while conserving resources.
Detailed Steps of DNA Replication
DNA replication unfolds in three main stages: initiation, elongation, and termination. Each step involves precise coordination to ensure fidelity.
Initiation of DNA Replication
This kickoff phase begins at specific sites called origins of replication. In prokaryotes, there’s usually one origin per chromosome, while eukaryotes have thousands to speed up the process for their larger genomes.
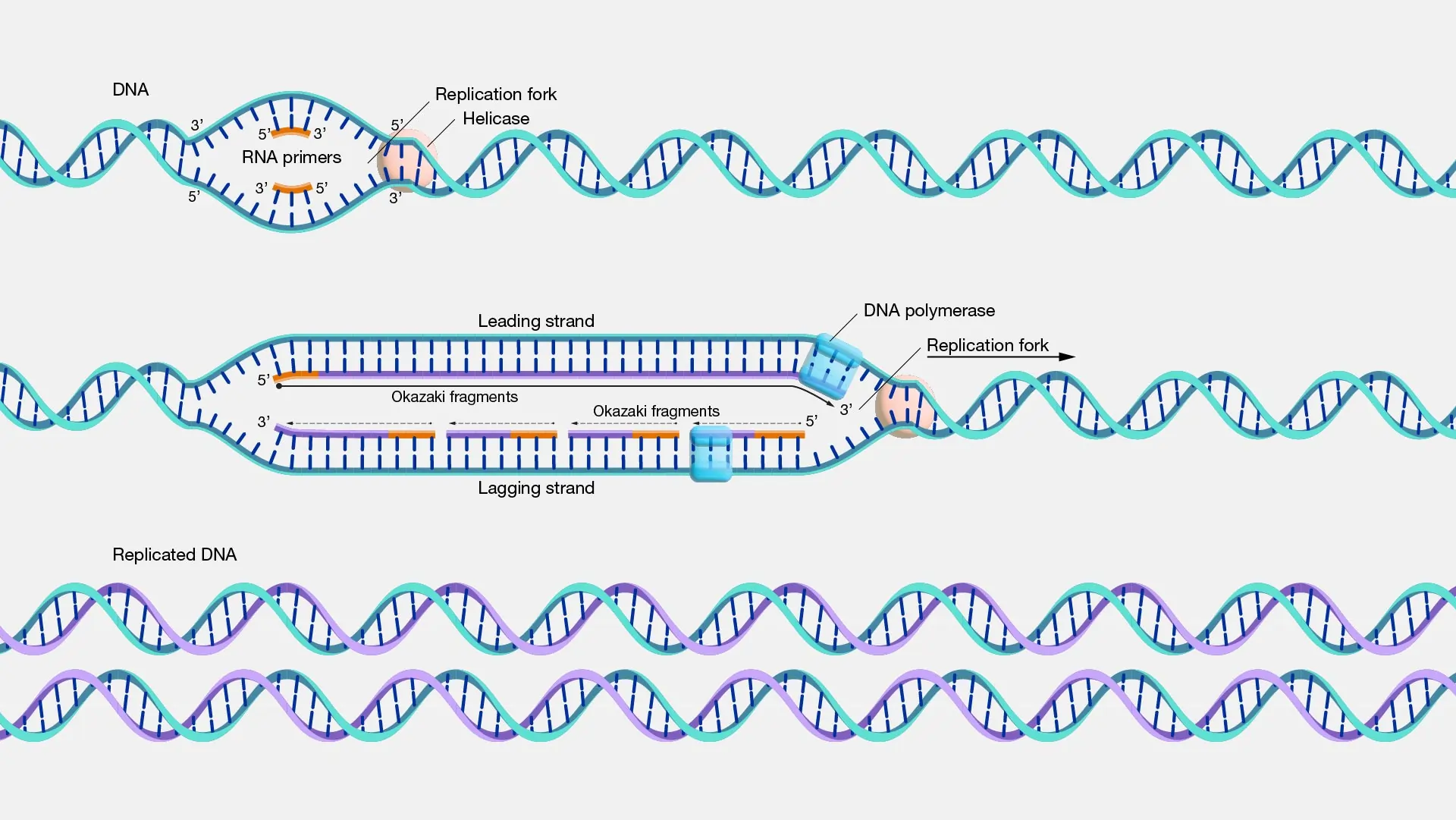
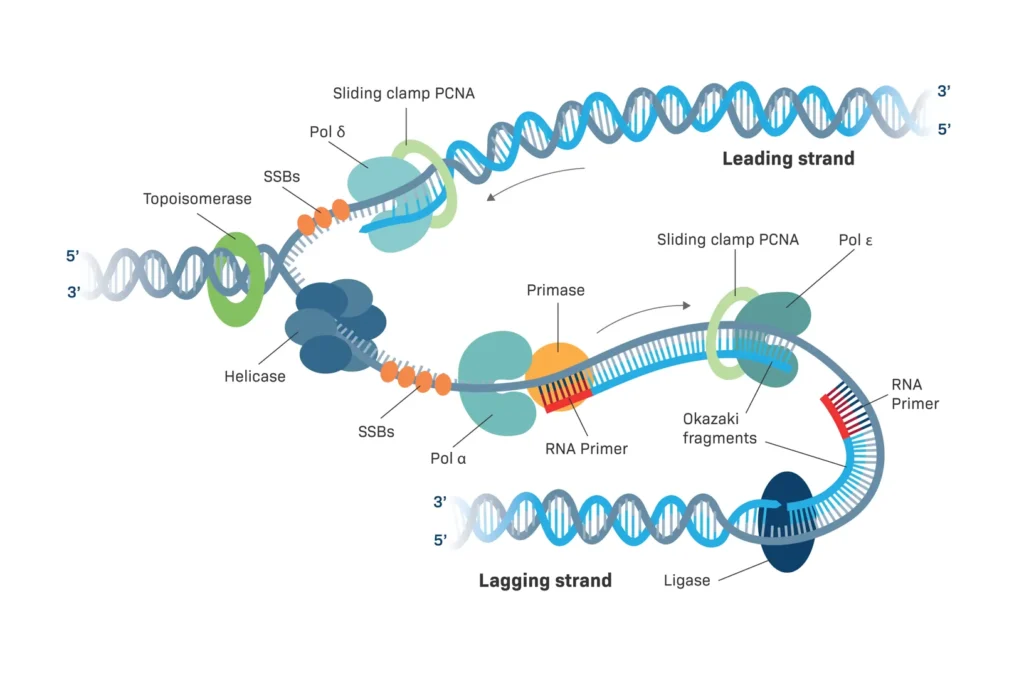
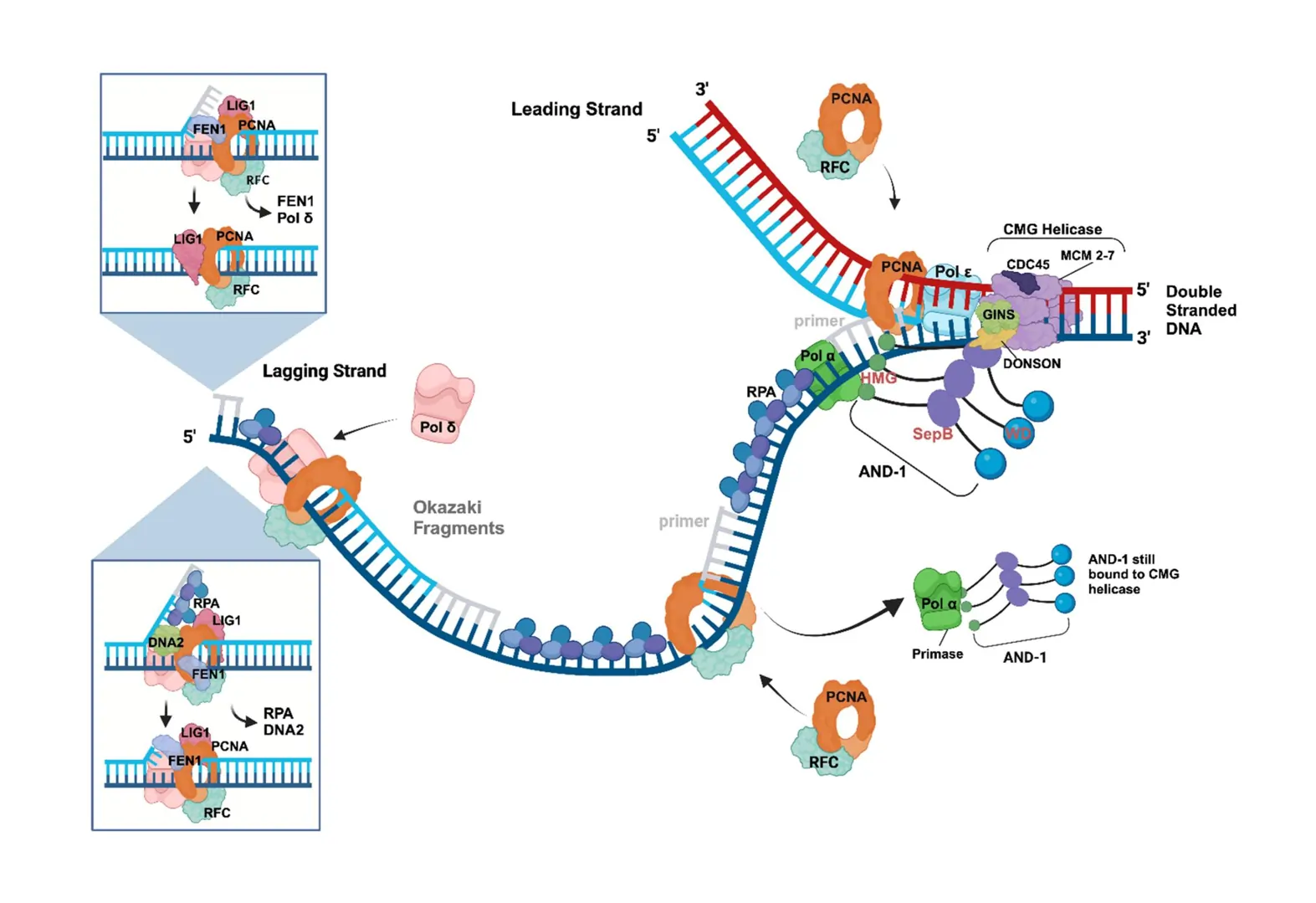
Enzymes like helicase bind to the origin, unwinding the double helix by breaking hydrogen bonds between bases. This creates a Y-shaped structure known as the replication fork. Topoisomerase relieves tension ahead of the fork by nicking and resealing strands, preventing tangles.
Next, primase synthesizes short RNA primers, providing a starting point for DNA synthesis since DNA polymerase can’t start from scratch. These primers are about 10 nucleotides long and complementary to the template.
In eukaryotes, initiation is tightly regulated by proteins like ORC (origin recognition complex) to prevent over-replication.
Elongation: Building the New Strands
Elongation is where the bulk of synthesis occurs. DNA polymerase adds nucleotides to the growing chain, matching them to the template: A to T, G to C.
Synthesis happens in the 5′ to 3′ direction, leading to two strands:
- Leading Strand: Synthesized continuously as the fork opens, following the helicase.
- Lagging Strand: Built discontinuously in short Okazaki fragments because it’s oriented oppositely. Each fragment starts with an RNA primer, and polymerase fills in backward.
Single-strand binding proteins (SSBPs) coat exposed single strands to prevent re-annealing or degradation.
As elongation proceeds, DNA polymerase I (in prokaryotes) removes RNA primers and replaces them with DNA, while ligase seals the nicks, creating a seamless strand.
This bidirectional process—forks moving in both directions from the origin—doubles efficiency.
Termination of DNA Replication
Termination wraps up when replication forks meet or reach chromosome ends. In circular prokaryotic DNA, forks converge, and ligase seals final gaps.
In linear eukaryotic chromosomes, telomeres—repetitive sequences at ends—protect against shortening. Telomerase adds repeats to maintain length, crucial for cell immortality in stem or cancer cells.
Post-termination, proofreading ensures accuracy, with error rates as low as one in a billion bases.
Key Enzymes in DNA Replication and Their Roles
Enzymes are the workhorses of replication, each with specialized functions.
Here’s a breakdown:
- DNA Helicase: Unwinds the double helix, creating single strands. In E. coli, it’s DnaB; in humans, MCM complex. It uses ATP to move along DNA, breaking bonds.
- DNA Polymerase: The star synthesizer. Prokaryotes have types I, II, III; eukaryotes α, δ, ε. They add nucleotides at 1000 per second in bacteria, with proofreading via 3′ to 5′ exonuclease activity.
- Topoisomerase: Relieves supercoiling. Type I cuts one strand; Type II both. Without it, DNA would knot like overtwisted rope.
- DNA Ligase: Joins Okazaki fragments by forming phosphodiester bonds, using ATP or NAD+.
- Primase: Makes RNA primers, essential for polymerase initiation.
- Endonucleases: Cleave internal bonds, aiding repair.
- Single-Strand Binding Proteins: Stabilize single strands, like scaffolding during construction.
These enzymes collaborate like a factory assembly line, ensuring precise duplication.
Prokaryotic DNA Polymerases: Types and Functions
In bacteria, polymerases vary:
- DNA Polymerase I: Repairs and removes primers, with 5′ to 3′ exonuclease.
- DNA Polymerase II: Backup for repair, 3′ to 5′ exonuclease.
- DNA Polymerase III: Main replicator, high processivity, adds thousands of nucleotides without falling off.
- DNA Polymerase IV and V: Involved in error-prone repair during stress.
Eukaryotic DNA Polymerases: Types and Functions
Eukaryotes have more specialized ones:
- DNA Polymerase α: Initiates with primase activity.
- DNA Polymerase δ: Elongates lagging strand, high fidelity.
- DNA Polymerase ε: Handles leading strand.
- DNA Polymerase γ: For mitochondrial DNA.
This specialization reflects the complexity of larger genomes.
DNA Replication Process in Prokaryotes
Prokaryotes, like E. coli, replicate efficiently due to simpler structure. Starting at oriC, helicase (DnaB) unwinds, SSBPs bind, primase (DnaG) primes.
Polymerase III synthesizes, with leading continuous and lagging in fragments. Polymerase I cleans up primers, ligase seals.
The whole 4.6 million base pair genome replicates in 40 minutes, showcasing speed.
Example: In bacterial infections, antibiotics target replication enzymes, halting growth.
DNA Replication in Eukaryotes
Eukaryotic replication is more intricate, with multiple origins activating in S phase. Licensing factors ensure once-per-cycle replication.
Polymerases δ and ε handle strands, with PCNA as a sliding clamp for processivity.
Telomere maintenance prevents “end replication problem,” where lagging strands shorten without telomerase.
In humans, replication takes hours for 6 billion bases, coordinated across chromosomes.
Example: In yeast, studies reveal how replication timing affects gene expression.
Differences Between Prokaryotic and Eukaryotic DNA Replication
While similar, key differences exist due to genome size and complexity.
| Aspect | Prokaryotes | Eukaryotes |
|---|---|---|
| Location | Cytoplasm | Nucleus (mitochondria separate) |
| Origins of Replication | Single (e.g., oriC in E. coli) | Multiple (thousands per chromosome) |
| Main DNA Polymerase | Pol III | Pol δ (lagging), Pol ε (leading) |
| Primer Removal | Pol I | Flap endonuclease and Pol δ |
| Okazaki Fragments | Shorter (1000-2000 nt) | Longer (100-200 nt) |
| Replication Speed | Faster (up to 1000 nt/sec) | Slower (50-100 nt/sec) |
| Chromosome Structure | Circular | Linear with telomeres |
| Regulation | Less complex, continuous | Tightly regulated by cell cycle checkpoints |
| Telomere Handling | Not needed (circular) | Telomerase adds repeats |
| Error Rate | Similar, ~10^-9 per base | Similar, with additional repair mechanisms |
This table highlights adaptations for larger, compartmentalized genomes.
Proofreading and Fidelity in DNA Replication
Fidelity ensures accuracy, with polymerases selecting correct nucleotides via shape and hydrogen bonding. Proofreading exonuclease removes mismatches immediately.
Mismatch repair systems scan post-replication, excising errors. In E. coli, MutS detects, MutH nicks, helicase unwinds, polymerase refills.
High fidelity keeps mutation rates low, but some slippage allows evolution. In viruses, high mutation rates aid adaptation, like in HIV.
Mutations and Genetic Variations from Replication Errors
Despite safeguards, errors occur: base substitutions, insertions, deletions. These mutations can be neutral, beneficial, or harmful.
For example, a point mutation in hemoglobin causes sickle cell anemia, but offers malaria resistance in carriers.
Replication stress from toxins can increase mutations, linking to cancer—e.g., BRCA mutations impair repair, raising breast cancer risk.
Over generations, mutations drive speciation, like antibiotic resistance in bacteria.
Real-World Examples and Applications
In forensics, DNA replication via PCR amplifies crime scene samples for profiling.
In gene therapy, understanding replication helps insert corrective genes without disrupting it.
Agriculture uses replication knowledge to engineer pest-resistant crops.
In aging research, telomere shortening from incomplete replication correlates with cellular senescence.
These applications show replication’s far-reaching impact, from health to innovation.
In summary, DNA replication is a symphony of molecular precision, essential for life’s continuity. By delving into its depths, we gain insights into our biological essence and potential futures.
Read These Articles in Detail
- The Cell: The Fundamental Building Block of Life
- Prokaryotic Cells vs. Eukaryotic Cells: A Detailed Exploration
- Prokaryotic Cells: Definition, Anatomy, Functions, and Their Importance
- Eukaryotic Cells Explained: Definition, Structure, Functions, and Importance
- The Cell Membrane: Structure, Functions, and Evolutionary Significance
- The Cell Wall: Structure, Composition, Functions, and Evolutionary Significance
- Endomembrane System: A Detailed Exploration of the Cellular Machinery of Life
- Mitochondria: A Comprehensive Guide to Their Structure and Functions
- The Cell Envelope: The Protective Shield of Prokaryotic Cells
- Ribosomes: Definition, Structure, and Functions
- Endoplasmic Reticulum: Structure, Types, and Functions Explained
- The Golgi Apparatus: Structure, Functions, and Cellular Importance
- Cytoplasm and Nucleus: Structure, Functions, Differences, and Examples
- What Is Cell Signaling? Structure, Types, and Functions Explained
- Three Main Parts of a Nucleotide: Structure and Functions
- Enzymes: Definition, Structure, Classification, and Functions
- DNA: Structure, Function, Discovery, and Modern Applications
- DNA Replication: Steps, Process, and Genetic Importance
- Properties of DNA: Structure, Physical, and Chemical Characteristics
- DNA Packaging: How DNA Fits into the Nucleus of a Cell
- R-Factors and Plasmids: How Bacteria Develop Antibiotic Resistance
- Chromosomes: Structure, Types, Functions, and Role in Genetics
- Genetic Material: DNA, RNA, and Their Role in Heredity and Evolution
- Deoxyribose and Ribose: Structure and Function in DNA and RNA
- Differences Between Genes and DNA: Definitions, Structures, and Functions
- RNA: Definition, Structure, Types, Functions, and Its Role in Life
Frequently Asked Questions
FAQ 1: What is DNA replication and why is it important?
DNA replication is the process where a cell makes an exact copy of its DNA, ensuring that genetic information is passed on accurately during cell division. This process is crucial because it allows cells to grow, repair tissues, and reproduce, maintaining the continuity of life. Without it, new cells wouldn’t have the instructions needed to function, leading to chaos in biological systems. It happens in the nucleus of eukaryotic cells (like those in humans) and in the cytoplasm of prokaryotic cells (like bacteria).
The importance of DNA replication extends beyond just cell division. It’s the foundation for passing traits from parents to offspring, like hair color or height, through the inheritance of genetic material. It also plays a role in repairing damaged DNA caused by things like UV rays, which helps prevent diseases like cancer. Additionally, small errors during replication can lead to mutations, which drive evolution by introducing genetic diversity. For example, a mutation might give a plant resistance to drought, helping its species survive in harsh climates.
In fields like biotechnology, understanding DNA replication has led to breakthroughs like PCR (polymerase chain reaction), a technique that copies tiny DNA samples for use in forensics or medical diagnostics. This process is a cornerstone of life, ensuring both stability and adaptability across generations.
FAQ 2: How does DNA replication work in simple terms?
DNA replication is like photocopying a massive instruction manual so that each new cell gets a complete copy. It starts when an enzyme called DNA helicase unzips the double helix, splitting the two strands of DNA. Each strand then acts as a template for building a new matching strand, making the process semi-conservative—each new DNA molecule has one old strand and one new one.
The process has three main steps: initiation, elongation, and termination. In initiation, specific spots on the DNA called origins of replication are opened up, and an enzyme called primase lays down short RNA primers to start the copying. During elongation, DNA polymerase adds matching nucleotides to build the new strands. One strand, called the leading strand, is built continuously, while the lagging strand is made in short pieces called Okazaki fragments. Finally, in termination, the replication finishes when the copying forks meet, and enzymes like DNA ligase seal any gaps.
This process is highly accurate, with proofreading mechanisms fixing most errors. For instance, in bacteria, replication of a 4.6 million base pair genome takes about 40 minutes, showing how efficient and precise it is.
FAQ 3: What are the key enzymes involved in DNA replication?
DNA replication relies on a team of enzymes, each with a specific job to ensure the process runs smoothly. DNA helicase starts by unwinding the double helix, breaking the hydrogen bonds between base pairs to create a replication fork. This was first discovered in E. coli in 1976 and is critical for exposing the DNA strands for copying.
DNA polymerase is the star player, adding nucleotides to form new strands while also proofreading to catch errors. In bacteria, DNA polymerase III does most of the replication, while DNA polymerase I removes RNA primers and fills gaps. In humans, DNA polymerase δ and DNA polymerase ε handle the main synthesis. Primase lays down RNA primers to give polymerase a starting point, since it can’t begin on its own. DNA ligase seals the gaps between Okazaki fragments, creating a continuous strand. Topoisomerase prevents the DNA from getting too twisted by making temporary nicks, and single-strand binding proteins (SSBPs) keep the unwound strands stable.
Each enzyme works like a specialized worker on an assembly line. For example, in rapidly dividing cells like those in the human gut, these enzymes coordinate to replicate billions of DNA bases accurately every day, ensuring cells function properly.
FAQ 4: What are the differences between prokaryotic and eukaryotic DNA replication?
Prokaryotic DNA replication (in bacteria) and eukaryotic DNA replication (in plants, animals, and fungi) share the same basic steps but differ due to genome size and cell structure. In prokaryotes, replication happens in the cytoplasm and usually starts at a single origin of replication. For example, in E. coli, the origin is called oriC, and the process is fast, copying 4.6 million base pairs in about 40 minutes.
In eukaryotes, replication occurs in the nucleus and involves multiple origins—thousands per chromosome—to handle larger genomes, like the 6 billion base pairs in humans. Eukaryotes use DNA polymerase δ and DNA polymerase ε for synthesis, while prokaryotes rely on DNA polymerase III. Eukaryotic chromosomes are linear, requiring telomerase to maintain telomeres (protective DNA ends), whereas prokaryotic DNA is circular and doesn’t need this.
Another difference is regulation. Eukaryotic replication is tightly controlled by cell cycle checkpoints to prevent errors, while prokaryotic replication is simpler and often continuous. These differences reflect the complexity of eukaryotic cells, which need to coordinate replication across many chromosomes in a confined space.
FAQ 5: What is the role of DNA polymerase in replication?
DNA polymerase is the enzyme responsible for building new DNA strands during replication. It reads the existing DNA strand (the template) and adds complementary nucleotides one by one, forming a new strand that matches the original. For example, if the template has an adenine (A), the polymerase adds a thymine (T). This happens in the 5′ to 3′ direction, ensuring accurate copying.
DNA polymerase also has a proofreading function, using its 3′ to 5′ exonuclease activity to remove incorrect nucleotides. In prokaryotes, DNA polymerase III is the main enzyme for replication, adding up to 1000 nucleotides per second with high accuracy. DNA polymerase I cleans up RNA primers and fills gaps. In eukaryotes, DNA polymerase δ and DNA polymerase ε handle the lagging and leading strands, respectively, while DNA polymerase α starts the process with primase activity.
This enzyme’s precision is vital. For instance, in cancer research, faulty DNA polymerase activity can lead to uncontrolled replication, highlighting its role in maintaining genetic stability.
FAQ 6: Why is DNA replication called semi-conservative?
DNA replication is called semi-conservative because each new DNA molecule contains one original strand from the parent DNA and one newly synthesized strand. This was proven in 1958 by the Meselson-Stahl experiment, where bacteria grown in heavy nitrogen showed that after replication, each daughter DNA had one heavy and one light strand, confirming the model.
Here’s how it works: the double helix unzips, and each single strand serves as a template for a new complementary strand. Enzymes like DNA polymerase build the new strand by matching bases (A with T, G with C). This ensures that half the original DNA is conserved in each new molecule, maintaining genetic fidelity.
This process is efficient and reduces errors, as the original strand acts as a reliable guide. It’s like keeping one page of a recipe book while copying the other, ensuring the instructions stay intact. This mechanism underpins genetic inheritance, allowing traits to pass accurately from cell to cell or parent to offspring.
FAQ 7: What are Okazaki fragments and their role in DNA replication?
Okazaki fragments are short segments of DNA synthesized on the lagging strand during replication. Because DNA polymerase can only add nucleotides in the 5′ to 3′ direction, the lagging strand, which runs opposite to the replication fork, is built in short bursts rather than continuously like the leading strand.
Here’s the process: primase lays down an RNA primer, and DNA polymerase adds nucleotides to form a fragment (100-200 nucleotides in eukaryotes, 1000-2000 in prokaryotes). These fragments are later joined by DNA ligase, which seals the gaps to create a continuous strand. Named after Reiji Okazaki, who discovered them in the 1960s, these fragments ensure that both strands are copied accurately despite their opposite orientations.
For example, in a dividing human cell, millions of Okazaki fragments are produced and stitched together to replicate the genome. This discontinuous synthesis is a clever solution to the directional challenge, ensuring every part of the DNA is duplicated.
FAQ 8: How does DNA replication prevent errors?
DNA replication is incredibly accurate, with error rates as low as one in a billion bases, thanks to multiple safeguards. The first is base pairing specificity, where DNA polymerase selects nucleotides that match the template (A with T, G with C) based on shape and hydrogen bonding. If a wrong nucleotide sneaks in, polymerase’s 3′ to 5′ exonuclease activity acts like an eraser, removing the mistake and trying again.
After replication, mismatch repair systems scan for errors. In bacteria, proteins like MutS detect mismatches, MutH cuts the faulty strand, and polymerase refills correctly. Eukaryotes have similar systems, like MSH2. Additionally, checkpoint proteins in eukaryotes monitor replication progress, pausing if issues arise.
For instance, in skin cells exposed to UV light, these mechanisms fix damage before it becomes a mutation, preventing issues like skin cancer. This layered approach ensures genetic stability, though rare errors can lead to beneficial diversity or harmful diseases.
FAQ 9: What happens if DNA replication goes wrong?
When DNA replication goes wrong, it can lead to mutations—changes in the DNA sequence like substitutions, insertions, or deletions. Most errors are caught by proofreading or repair systems, but those that slip through can have varied effects. Some mutations are harmless, like a silent mutation that doesn’t change a protein’s function. Others, like a mutation in the BRCA1 gene, can increase cancer risk by impairing repair mechanisms.
In severe cases, replication errors cause genetic disorders. For example, a mutation in the hemoglobin gene leads to sickle cell anemia, altering red blood cells’ shape. However, this same mutation can protect against malaria, showing how errors can sometimes be beneficial. In cancer, uncontrolled replication due to faulty checkpoints allows tumors to grow rapidly.
Environmental factors like radiation or chemicals can increase error rates, stressing replication. Understanding these risks helps scientists develop therapies, like drugs that target cancer cells’ replication machinery, to stop their growth while sparing healthy cells.
FAQ 10: How is DNA replication used in real-world applications?
DNA replication principles are applied in many fields, transforming science and medicine. In forensics, PCR mimics replication to amplify tiny DNA samples from crime scenes, enabling identification through genetic profiling. This has solved countless cases by matching DNA with suspects or victims.
In medicine, replication knowledge aids gene therapy, where correct genes are inserted into cells to treat disorders like cystic fibrosis. By understanding how DNA polymerase works, scientists ensure these genes integrate without disrupting replication. In biotechnology, replication is used to clone genes for research or produce proteins like insulin in bacteria.
In agriculture, replication insights help engineer crops with desirable traits, like pest resistance, by modifying DNA. In evolutionary biology, studying replication errors reveals how species adapt, like antibiotic-resistant bacteria. Even in aging research, scientists explore how telomere shortening during replication contributes to cellular aging, paving the way for anti-aging therapies. These applications show how a cellular process shapes modern innovation.
FAQ 11: What is the history behind the discovery of DNA replication?
The journey to understanding DNA replication began in the early 1950s, right after James Watson and Francis Crick unveiled the double-helix structure of DNA in 1953. Their model suggested that the complementary base pairing could explain how DNA copies itself, but it was just a hypothesis at the time. Scientists were eager to figure out the exact mechanism, leading to a flurry of experiments. One key milestone was the work on how DNA unwinds and rebuilds, but the real breakthrough came with proving the replication model.
In 1958, Matthew Meselson and Franklin Stahl conducted what many call the most beautiful experiment in biology. They used bacteria grown in a medium with heavy nitrogen isotopes to label the DNA, then switched to light nitrogen and observed how the DNA density changed over generations. This pulse-chase method showed that DNA replication is semi-conservative, with each new molecule containing one old and one new strand. Their findings, published in a landmark paper, solidified the Watson-Crick model and opened doors to molecular biology. Before this, ideas floated around, but Meselson and Stahl’s elegant density gradient centrifugation provided concrete evidence.
Further discoveries built on this. In the 1960s, enzymes like DNA polymerase were isolated, with Arthur Kornberg earning a Nobel for his work on bacterial replication. The 1970s saw the identification of helicase in E. coli, revealing how the helix unzips. By the 1980s, eukaryotic replication complexities, like multiple origins, were mapped. These steps weren’t just academic; they influenced fields like genetics and medicine, helping explain mutations and heredity.
Today, the history of DNA replication reminds us of science’s collaborative nature. From Watson and Crick’s structure to modern imaging techniques watching replication in real time, it’s a story of curiosity driving progress. This evolution in knowledge continues to inspire research into diseases linked to replication errors, like cancer.
FAQ 12: What are the different models of DNA replication?
Scientists once debated how DNA duplicates itself, proposing three main models: conservative, semi-conservative, and dispersive. Each offered a unique way to explain how genetic material passes on, but experiments ultimately favored one.
| Model | Description | Key Features | Evidence Against or For | Example in Nature |
|---|---|---|---|---|
| Conservative Replication | The original DNA molecule remains intact, serving as a template to create a completely new copy. The parent DNA is conserved entirely, and the daughter is brand new. | No mixing of strands; parent and daughter separate after replication. | Disproven by Meselson-Stahl experiment, which showed hybrid DNA after one generation, not separate heavy and light molecules. | Rarely seen, but similar to some plasmid replications in bacteria. |
| Semi-Conservative Replication | The double helix unwinds, and each strand acts as a template for a new complementary strand, resulting in two molecules each with one old and one new strand. | Efficient and accurate; conserves half the original material per molecule. | Supported by Meselson-Stahl’s density labeling, showing intermediate density DNA initially, then splitting further. | Universal in cells, from bacteria to humans, ensuring genetic stability. |
| Dispersive Replication | The original DNA breaks into fragments, and new pieces fill in, mixing old and new segments throughout both daughter molecules. | Results in hybrid strands with scattered parental bits. | Ruled out because experiments showed clear banding in density gradients, not a uniform mix. | Not observed in standard replication, but echoes some repair processes. |
These models highlight early uncertainties in molecular biology. The semi-conservative model, proven in 1958, became the foundation for understanding inheritance and evolution. It explains why traits persist accurately while allowing for variations through mutations.
FAQ 13: What is a replication fork and what are its components?
The replication fork is a crucial structure in DNA replication, resembling a Y-shape where the double helix splits to allow copying. It forms when helicase unwinds the DNA, creating two single strands that serve as templates. This dynamic area moves along the chromosome, enabling efficient duplication.
Understanding its components helps grasp how replication stays accurate and speedy. Here’s a breakdown:
- Helicase: Acts like a zipper opener, breaking hydrogen bonds to separate strands and advance the fork.
- Single-Strand Binding Proteins (SSBPs): Coat exposed strands to prevent re-annealing or damage, keeping them stable for synthesis.
- Topoisomerase: Relieves supercoiling ahead of the fork by nicking and resealing DNA, avoiding tangles.
- Primase: Synthesizes RNA primers to start DNA polymerase on both strands.
- DNA Polymerase: Builds new strands by adding nucleotides; works continuously on the leading strand and in fragments on the lagging.
- Ligase: Seals gaps between Okazaki fragments on the lagging strand.
These parts work together like a coordinated team. For instance, in human cells, multiple forks activate to replicate the vast genome quickly. Problems at the fork, like stalling, can lead to mutations, linking to diseases.
FAQ 14: How do telomeres and telomerase play a role in DNA replication?
Telomeres are protective caps at the ends of linear chromosomes, made of repetitive DNA sequences that safeguard the genetic code from erosion during replication. Each time a cell divides, DNA polymerase can’t fully copy the ends on the lagging strand, causing shortening. Telomeres act as a buffer, absorbing this loss so vital genes aren’t affected.
Telomerase, an enzyme with an RNA template, counters this by adding repeats to telomere ends. It functions as a reverse transcriptase, extending the 3′ end to maintain length. In most somatic cells, telomerase is inactive, leading to gradual shortening and eventual cell senescence, a natural aging process. But in stem cells and cancer cells, it’s active, allowing indefinite division.
This mechanism solves the end replication problem, ensuring chromosome stability. Without it, ends could fuse or degrade, causing genomic chaos. Research links short telomeres to age-related diseases like heart conditions, while overactive telomerase drives 90% of cancers. Future therapies might target telomerase to fight cancer or extend healthy lifespan.
FAQ 15: How does DNA replication work in viruses?
Viruses hijack host machinery for replication, but DNA viruses have unique strategies. They enter cells, uncoating their genome, then use host or viral enzymes to copy DNA. For example, herpesviruses replicate in the nucleus, forming replication compartments.
Key aspects include:
- Nuclear Replication: Most DNA viruses, like adenoviruses, replicate in the host nucleus, borrowing polymerases but encoding their own for efficiency.
- Rolling Circle Mechanism: Some, like bacteriophages, use this for rapid copying, producing long concatamers later cut into genomes.
- Bidirectional Forks: Similar to cells, but viruses optimize for speed, like hepatitis B using reverse transcriptase despite being DNA-based.
- Assembly and Release: New genomes package into capsids; enveloped viruses bud from membranes.
In HIV, though RNA, it integrates as DNA via reverse transcriptase. Understanding viral replication aids antiviral development, like drugs blocking polymerase.
FAQ 16: What are the applications of DNA replication in medicine and biotechnology?
DNA replication principles fuel innovations in health and tech, from diagnostics to therapies.
| Application | Description | Benefits | Examples | Challenges |
|---|---|---|---|---|
| PCR Technology | Amplifies DNA segments mimicking replication for analysis. | Rapid, sensitive detection. | COVID testing, forensics. | Contamination risks. |
| Gene Therapy | Inserts corrective genes, relying on replication integration. | Treats genetic disorders. | Cystic fibrosis trials. | Delivery efficiency. |
| Cancer Treatments | Targets faulty replication in tumors, like inhibiting polymerases. | Selective killing of cancer cells. | Chemotherapy drugs. | Side effects on normal cells. |
| Synthetic Biology | Engineers organisms by manipulating replication. | Produces biofuels, medicines. | Insulin from bacteria. | Ethical concerns. |
| Cloning | Replicates DNA for organism duplication. | Advances research, agriculture. | Dolly the sheep. | Low success rates. |
These uses transform lives, but ethical oversight is key.
FAQ 17: What are some common misconceptions about DNA replication?
Many people mix up DNA replication with cell division, thinking they’re the same. Replication happens in the S phase, preparing DNA before mitosis or meiosis divides the cell. Without it, division couldn’t occur properly.
Other myths include:
- One Polymerase per Strand: Actually, multiple polymerases work, with teams on lagging strands for Okazaki fragments.
- Error-Free Process: While accurate, mutations happen, driving evolution but also diseases.
- Single Origin Only: Eukaryotes have thousands for efficiency.
- Sense and Template Strands Confusion: The template is antisense; sense matches mRNA.
Clearing these helps appreciate replication’s complexity.
FAQ 18: What is the difference between DNA replication and transcription?
DNA replication creates identical DNA copies for cell division, ensuring genetic continuity. It uses the entire genome as a template, producing two double-stranded molecules via semi-conservative means. Enzymes like polymerase add deoxyribonucleotides, matching A-T and G-C.
Transcription, however, copies DNA into RNA for protein synthesis. It targets specific genes, producing single-stranded RNA using uracil instead of thymine. RNA polymerase drives this, unwinding DNA temporarily without primers. Replication is bidirectional from origins; transcription unidirectional from promoters.
Replication occurs once per cycle in the nucleus; transcription ongoing as needed. Errors in replication cause mutations; in transcription, faulty mRNA. Both are vital, but replication maintains the blueprint, transcription expresses it.
FAQ 19: What is the evolutionary significance of DNA replication?
DNA replication’s fidelity ensures species stability over generations, passing traits accurately. Yet, its slight error rate introduces mutations, fueling natural selection and diversity. Early life likely used RNA, but DNA’s stability offered advantages, evolving complex machineries.
In bacteria and archaea, replication systems diverged, suggesting independent evolution in lineages. This plasticity allows adaptation, like in cancer where replication stress drives evolution. Replication origins influence genome structure, affecting expression and evolution.
Overall, it’s a balance: precision for conservation, variability for change, shaping life’s tree.
FAQ 20: What does future research hold for DNA replication?
Future studies aim to unravel replication’s intricacies, potentially revolutionizing medicine. Advances in imaging will watch forks in real time, revealing stress responses.
Promising areas include:
- Synthetic DNA: Creating artificial systems for gene therapy, mimicking replication.
- Cancer Targets: Drugs hitting replication enzymes in tumors.
- Aging and Telomeres: Extending healthy life by manipulating shortening.
- Single-Cell Dynamics: Tracking variability in cells for personalized medicine.
These could lead to breakthroughs in disease prevention.
Acknowledgement
The creation of “DNA Replication: Steps, Process, and Genetic Importance” was made possible through the wealth of knowledge available from various reputable sources. The Examsmeta.com Website expresses its gratitude to the scientific community and educational platforms that provide accessible, high-quality information on molecular biology.
Specifically, acknowledges the contributions of Nature (www.nature.com) for its extensive research articles, ScienceDirect (www.sciencedirect.com) for its detailed scientific publications, PubMed (pubmed.ncbi.nlm.nih.gov) for its comprehensive database of biomedical literature, Khan Academy (www.khanacademy.org) for its clear educational resources, and NCBI (www.ncbi.nlm.nih.gov) for its invaluable genetic and molecular biology insights. These platforms provided critical references that enriched the article’s depth and accuracy.

